
In chemistry, alcohol is an organic compound that carries at least one hydroxyl functional group (−OH) bound to a saturated carbon atom. The term alcohol originally referred to the primary alcohol ethanol (ethyl alcohol), which is used as a drug and is the main alcohol present in alcoholic drinks. An important class of alcohols, of which methanol and ethanol are the simplest members, includes all compounds for which the general formula is CnH2n+1OH. Simple monoalcohols that are the subject of this article include primary (RCH2OH), secondary (R2CHOH) and tertiary (R3COH) alcohols.

In chemistry, a ketone is a functional group with the structure R2C=O, where R can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond). The simplest ketone is acetone (R = R' = methyl), with the formula CH3C(O)CH3. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone.
Chemically, an aldehyde is a compound containing a functional group with the structure −CHO, consisting of a carbonyl center with the carbon atom also bonded to hydrogen and to any generic alkyl or side chain R group. The functional group itself is known as an aldehyde or formyl group.

Tartaric acid is a white, crystalline organic acid that occurs naturally in many fruits, most notably in grapes, but also in bananas, tamarinds, and citrus. Its salt, potassium bitartrate, commonly known as cream of tartar, develops naturally in the process of fermentation. It is commonly mixed with sodium bicarbonate and is sold as baking powder used as a leavening agent in food preparation. The acid itself is added to foods as an antioxidant E334 and to impart its distinctive sour taste.
In chemistry, a hydration reaction is a chemical reaction in which a substance combines with water. In organic chemistry, water is added to an unsaturated substrate, which is usually an alkene or an alkyne. This type of reaction is employed industrially to produce ethanol, isopropanol, and butan-2-ol.

Ethylene oxide is an organic compound with the formula C
2H
4O. It is a cyclic ether and the simplest epoxide: a three-membered ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless and flammable gas with a faintly sweet odor. Because it is a strained ring, ethylene oxide easily participates in a number of addition reactions that result in ring-opening. Ethylene oxide is isomeric with acetaldehyde and with vinyl alcohol. Ethylene oxide is industrially produced by oxidation of ethylene in the presence of silver catalyst.

Iron(III) chloride is the inorganic compound with the formula. Also called ferric chloride, it is a common compound of iron in the +3 oxidation state. The anhydrous compound is a crystalline solid with a melting point of 307.6 °C. The color depends on the viewing angle: by reflected light the crystals appear dark green, but by transmitted light they appear purple-red.

A dicarbonyl is a molecule containing two carbonyl (C=O) groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls are in close enough proximity that their reactivity is changed, such as 1,2-, 1,3-, and 1,4-dicarbonyls. Their properties often differ from those of monocarbonyls, and so they are usually considered functional groups of their own. These compounds can have symmetrical or unsymmetrical substituents on each carbonyl, and may also be functionally symmetrical or unsymmetrical.
A diol is a chemical compound containing two hydroxyl groups. An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified.

Rhodium(III) chloride refers to inorganic compounds with the formula RhCl3(H2O)n, where n varies from 0 to 3. These are diamagnetic solids featuring octahedral Rh(III) centres. Depending on the value of n, the material is either a dense brown solid or a soluble reddish salt. The soluble trihydrated (n = 3) salt is widely used to prepare compounds used in homogeneous catalysis, notably for the industrial production of acetic acid and hydroformylation.

Methyl methacrylate (MMA) is an organic compound with the formula CH2=C(CH3)COOCH3. This colorless liquid, the methyl ester of methacrylic acid (MAA), is a monomer produced on a large scale for the production of poly(methyl methacrylate) (PMMA).
Chloral, also known as trichloroacetaldehyde or trichloroethanal, is the organic compound with the formula Cl3CCHO. This aldehyde is a colourless oily liquid that is soluble in a wide range of solvents. It reacts with water to form chloral hydrate, a once widely used sedative and hypnotic substance.
Glyoxal is an organic compound with the chemical formula OCHCHO. It is the smallest dialdehyde. It is a crystalline solid, white at low temperatures and yellow near the melting point (15 °C). The liquid is yellow, and the vapor is green.
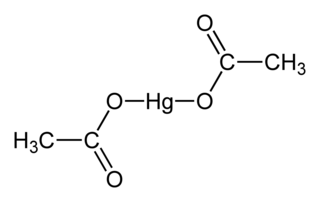
Mercury(II) acetate is the chemical compound with the formula Hg(O2CCH3)2. Commonly abbreviated Hg(OAc)2, this compound is employed as a reagent to generate organomercury compounds from unsaturated organic precursors. It is a white water-soluble solid, but samples appear yellowish with time owing to decomposition.
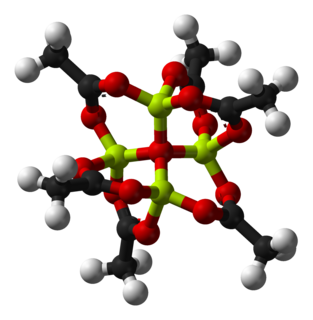
Basic beryllium acetate is the chemical compound with the formula Be4O(O2CCH3)6. This compound adopts a distinctive structure, but it has no applications and has been only lightly studied. It is a colourless solid that is soluble in organic solvents.

Aconitic acid is an organic acid. The two isomers are cis-aconitic acid and trans-aconitic acid. The conjugate base of cis-aconitic acid, cis-aconitate is an intermediate in the isomerization of citrate to isocitrate in the citric acid cycle. It is acted upon by the enzyme aconitase.
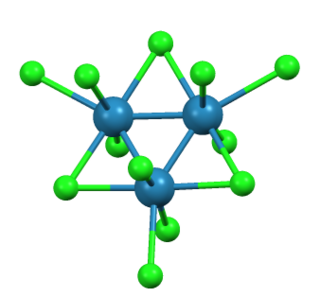
Trirhenium nonachloride is a compound with the formula ReCl3, sometimes also written Re3Cl9. It is a dark red hygroscopic solid that is insoluble in ordinary solvents. The compound is important in the history of inorganic chemistry as an early example of a cluster compound with metal-metal bonds. It is used as a starting material for synthesis of other rhenium complexes.

Bromopentacarbonylrhenium(I) is an inorganic compound of rhenium, commonly used for the syntheses of other rhenium complexes.

Methyl propionate, also known as methyl propanoate, is the organic compound with the molecular formula CH3CH2CO2CH3. It is a colorless liquid with a fruity, rum-like odor.

Diethylsuccinoylsuccinate is an organic compound with the formula [CH2C(OH)=C(CO2Et)]2 (Et = ethyl). A tetrasubstituted derivative of 1,4-cyclohexadiene, the compound is the enol tautomer of the corresponding cyclohexadione. It is produced by base-induced condensation of diethyl succinate:
















