
5α-Reductases, also known as 3-oxo-5α-steroid 4-dehydrogenases, are enzymes involved in steroid metabolism. They participate in three metabolic pathways: bile acid biosynthesis, androgen and estrogen metabolism. There are three isozymes of 5α-reductase encoded by the genes SRD5A1, SRD5A2, and SRD5A3.

Tolfenamic acid is a member of the anthranilic acid derivatives class of NSAID drugs. Like other members of the class, it is a COX inhibitor and prevents formation of prostaglandins.
17β-Hydroxysteroid dehydrogenases, also 17-ketosteroid reductases (17-KSR), are a group of alcohol oxidoreductases which catalyze the reduction of 17-ketosteroids and the dehydrogenation of 17β-hydroxysteroids in steroidogenesis and steroid metabolism. This includes interconversion of DHEA and androstenediol, androstenedione and testosterone, and estrone and estradiol.

Cyclooxygenase-2 (COX-2), also known as prostaglandin-endoperoxide synthase 2 (HUGO PTGS2), is an enzyme that in humans is encoded by the PTGS2 gene. In humans it is one of three cyclooxygenases. It is involved in the conversion of arachidonic acid to prostaglandin H2, an important precursor of prostacyclin, which is expressed in inflammation.

Retinoid X receptor alpha (RXR-alpha), also known as NR2B1 is a nuclear receptor that in humans is encoded by the RXRA gene.
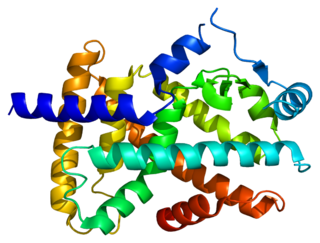
Peroxisome proliferator-activated receptor alpha (PPAR-α), also known as NR1C1, is a nuclear receptor protein functioning as a transcription factor that in humans is encoded by the PPARA gene. Together with peroxisome proliferator-activated receptor delta and peroxisome proliferator-activated receptor gamma, PPAR-alpha is part of the subfamily of peroxisome proliferator-activated receptors. It was the first member of the PPAR family to be cloned in 1990 by Stephen Green and has been identified as the nuclear receptor for a diverse class of rodent hepatocarcinogens that causes proliferation of peroxisomes.

COUP-TFII, also known as NR2F2 is a protein that in humans is encoded by the NR2F2 gene. The COUP acronym stands for chicken ovalbumin upstream promoter.

In enzymology, a β-ketoacyl-[acyl-carrier-protein] synthase III (EC 2.3.1.180) is an enzyme that catalyzes the chemical reaction

G protein-coupled receptor family C group 6 member A (GPRC6A) is a protein that in humans is encoded by the GPRC6A gene. This protein functions as a receptor of L-α-amino acids, cations, osteocalcin, and steroids. It is a membrane androgen receptor.

Acyl-CoA-binding protein in humans belongs to the family of Acyl-CoA-binding proteins.

E3 SUMO-protein ligase PIAS1 is an enzyme that in humans is encoded by the PIAS1 gene.

E3 SUMO-protein ligase PIAS2 is an enzyme that in humans is encoded by the PIAS2 gene.

25-hydroxycholesterol 7-alpha-hydroxylase also known as oxysterol and steroid 7-alpha-hydroxylase is an enzyme that in humans is encoded by the CYP7B1 gene. This gene encodes a member of the cytochrome P450 superfamily of enzymes. The cytochrome P450 proteins are monooxygenases which catalyze many reactions involved in drug metabolism and synthesis of cholesterol, steroids and other lipids.

A depside is a type of polyphenolic compound composed of two or more monocyclic aromatic units linked by an ester group. Depsides are most often found in lichens, but have also been isolated from higher plants, including species of the Ericaceae, Lamiaceae, Papaveraceae and Myrtaceae.

Biochanin A is an O-methylated isoflavone. It is a natural organic compound in the class of phytochemicals known as flavonoids. Biochanin A can be found in red clover in soy, in alfalfa sprouts, in peanuts, in chickpea and in other legumes.

Dynamic combinatorial chemistry (DCC); also known as constitutional dynamic chemistry (CDC) is a method to the generation of new molecules formed by reversible reaction of simple building blocks under thermodynamic control. The library of these reversibly interconverting building blocks is called a dynamic combinatorial library (DCL). All constituents in a DCL are in equilibrium, and their distribution is determined by their thermodynamic stability within the DCL. The interconversion of these building blocks may involve covalent or non-covalent interactions. When a DCL is exposed to an external influence, the equilibrium shifts and those components that interact with the external influence are stabilised and amplified, allowing more of the active compound to be formed.

Amentoflavone is a biflavonoid constituent of a number of plants including Ginkgo biloba, Chamaecyparis obtusa (hinoki), Biophytum sensitivum, Selaginella tamariscina, Hypericum perforatum and Xerophyta plicata.
A steroidogenesis inhibitor, also known as a steroid biosynthesis inhibitor, is a type of drug which inhibits one or more of the enzymes that are involved in the process of steroidogenesis, the biosynthesis of endogenous steroids and steroid hormones. They may inhibit the production of cholesterol and other sterols, sex steroids such as androgens, estrogens, and progestogens, corticosteroids such as glucocorticoids and mineralocorticoids, and neurosteroids. They are used in the treatment of a variety of medical conditions that depend on endogenous steroids.

11α-Hydroxyprogesterone (11α-OHP), or 11α-hydroxypregn-4-ene-3,20-dione is an endogenous steroid and metabolite of progesterone. It is a weak antiandrogen, and is devoid of androgenic, estrogenic, and progestogenic activity.

















