Related Research Articles

Isotope analysis is the identification of isotopic signature, abundance of certain stable isotopes of chemical elements within organic and inorganic compounds. Isotopic analysis can be used to understand the flow of energy through a food web, to reconstruct past environmental and climatic conditions, to investigate human and animal diets, for food authentification, and a variety of other physical, geological, palaeontological and chemical processes. Stable isotope ratios are measured using mass spectrometry, which separates the different isotopes of an element on the basis of their mass-to-charge ratio.

Magnesite is a mineral with the chemical formula MgCO
3. Iron, manganese, cobalt, and nickel may occur as admixtures, but only in small amounts.
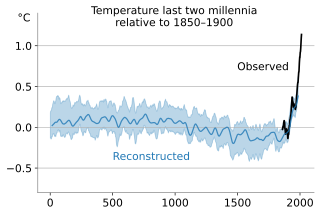
In the study of past climates ("paleoclimatology"), climate proxies are preserved physical characteristics of the past that stand in for direct meteorological measurements and enable scientists to reconstruct the climatic conditions over a longer fraction of the Earth's history. Reliable global records of climate only began in the 1880s, and proxies provide the only means for scientists to determine climatic patterns before record-keeping began.
In chemistry, isotopologues are molecules that differ only in their isotopic composition. They have the same chemical formula and bonding arrangement of atoms, but at least one atom has a different number of neutrons than the parent.
Kinetic fractionation is an isotopic fractionation process that separates stable isotopes from each other by their mass during unidirectional processes. Biological processes are generally unidirectional and are very good examples of "kinetic" isotope reactions. All organisms preferentially use lighter isotopes, because "energy costs" are lower, resulting in a significant fractionation between the substrate (heavier) and the biologically mediated product (lighter). For example, photosynthesis preferentially takes up the light isotope of carbon 12C during assimilation of atmospheric CO2. This kinetic isotope fractionation explains why plant material (and thus fossil fuels, which are derived from plants) is typically depleted in 13C by 25 per mil (2.5%) relative to most inorganic carbon on Earth.
A paleothermometer is a methodology that provides an estimate of the ambient temperature at the time of formation of a natural material. Most paleothermometers are based on empirically-calibrated proxy relationships, such as the tree ring or TEX86 methods. Isotope methods, such as the δ18O method or the clumped-isotope method, are able to provide, at least in theory, direct measurements of temperature.

Oxygen isotope ratio cycles are cyclical variations in the ratio of the abundance of oxygen with an atomic mass of 18 to the abundance of oxygen with an atomic mass of 16 present in some substances, such as polar ice or calcite in ocean core samples, measured with the isotope fractionation. The ratio is linked to ancient ocean temperature which in turn reflects ancient climate. Cycles in the ratio mirror climate changes in the geological history of Earth.
In geochemistry, paleoclimatology and paleoceanography δ18O or delta-O-18 is a measure of the deviation in ratio of stable isotopes oxygen-18 (18O) and oxygen-16 (16O). It is commonly used as a measure of the temperature of precipitation, as a measure of groundwater/mineral interactions, and as an indicator of processes that show isotopic fractionation, like methanogenesis. In paleosciences, 18O:16O data from corals, foraminifera and ice cores are used as a proxy for temperature.
Equilibrium isotope fractionation is the partial separation of isotopes between two or more substances in chemical equilibrium. Equilibrium fractionation is strongest at low temperatures, and forms the basis of the most widely used isotopic paleothermometers : D/H and 18O/16O records from ice cores, and 18O/16O records from calcium carbonate. It is thus important for the construction of geologic temperature records. Isotopic fractionations attributed to equilibrium processes have been observed in many elements, from hydrogen (D/H) to uranium (238U/235U). In general, the light elements are most susceptible to fractionation, and their isotopes tend to be separated to a greater degree than heavier elements.
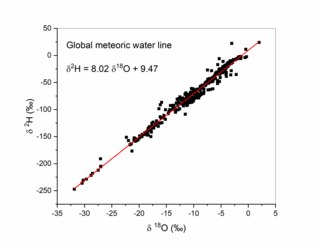
The Global Meteoric Water Line (GMWL) describes the global annual average relationship between hydrogen and oxygen isotope (oxygen-18 [18O] and deuterium [2H]) ratios in natural meteoric waters. The GMWL was first developed in 1961 by Harmon Craig, and has subsequently been widely used to track water masses in environmental geochemistry and hydrogeology.

Magmatic water, also known as juvenile water, is an aqueous phase in equilibrium with minerals that have been dissolved by magma deep within the Earth's crust and is released to the atmosphere during a volcanic eruption. It plays a key role in assessing the crystallization of igneous rocks, particularly silicates, as well as the rheology and evolution of magma chambers. Magma is composed of minerals, crystals and volatiles in varying relative natural abundance. Magmatic differentiation varies significantly based on various factors, most notably the presence of water. An abundance of volatiles within magma chambers decreases viscosity and leads to the formation of minerals bearing halogens, including chloride and hydroxide groups. In addition, the relative abundance of volatiles varies within basaltic, andesitic, and rhyolitic magma chambers, leading to some volcanoes being exceedingly more explosive than others. Magmatic water is practically insoluble in silicate melts but has demonstrated the highest solubility within rhyolitic melts. An abundance of magmatic water has been shown to lead to high-grade deformation, altering the amount of δ18O and δ2H within host rocks.
Hydrogen isotope biogeochemistry (HIBGC) is the scientific study of biological, geological, and chemical processes in the environment using the distribution and relative abundance of hydrogen isotopes. Hydrogen has two stable isotopes, protium 1H and deuterium 2H, which vary in relative abundance on the order of hundreds of permil. The ratio between these two species can be called the hydrogen isotopic signature of a substance. Understanding isotopic fingerprints and the sources of fractionation that lead to variation between them can be applied to address a diverse array of questions ranging from ecology and hydrology to geochemistry and paleoclimate reconstructions. Since specialized techniques are required to measure natural hydrogen isotopic composition (HIC), HIBGC provides uniquely specialized tools to more traditional fields like ecology and geochemistry.
Isotopic reference materials are compounds with well-defined isotopic compositions and are the ultimate sources of accuracy in mass spectrometric measurements of isotope ratios. Isotopic references are used because mass spectrometers are highly fractionating. As a result, the isotopic ratio that the instrument measures can be very different from that in the sample's measurement. Moreover, the degree of instrument fractionation changes during measurement, often on a timescale shorter than the measurement's duration, and can depend on the characteristics of the sample itself. By measuring a material of known isotopic composition, fractionation within the mass spectrometer can be removed during post-measurement data processing. Without isotope references, measurements by mass spectrometry would be much less accurate and could not be used in comparisons across different analytical facilities. Due to their critical role in measuring isotope ratios, and in part, due to historical legacy, isotopic reference materials define the scales on which isotope ratios are reported in the peer-reviewed scientific literature.

Position-specific isotope analysis, also called site-specific isotope analysis, is a branch of isotope analysis aimed at determining the isotopic composition of a particular atom position in a molecule. Isotopes are elemental variants with different numbers of neutrons in their nuclei, thereby having different atomic masses. Isotopes are found in varying natural abundances depending on the element; their abundances in specific compounds can vary from random distributions due to environmental conditions that act on the mass variations differently. These differences in abundances are called "fractionations," which are characterized via stable isotope analysis.
Shuhei Ono is a professor of earth, atmospheric, and planetary sciences at the Massachusetts Institute of Technology. In his research, he measures isotopes of sulfur and other elements to investigate water-rock-microbe interactions, seafloor hydrothermal systems, the deep biosphere, and global sulfur cycles.
Carbonate-associated sulfates (CAS) are sulfate species found in association with carbonate minerals, either as inclusions, adsorbed phases, or in distorted sites within the carbonate mineral lattice. It is derived primarily from dissolved sulfate in the solution from which the carbonate precipitates. In the ocean, the source of this sulfate is a combination of riverine and atmospheric inputs, as well as the products of marine hydrothermal reactions and biomass remineralisation. CAS is a common component of most carbonate rocks, having concentrations in the parts per thousand within biogenic carbonates and parts per million within abiogenic carbonates. Through its abundance and sulfur isotope composition, it provides a valuable record of the global sulfur cycle across time and space.
Methane clumped isotopes are methane molecules that contain two or more rare isotopes. Methane (CH4) contains two elements, carbon and hydrogen, each of which has two stable isotopes. For carbon, 98.9% are in the form of carbon-12 (12C) and 1.1% are carbon-13 (13C); while for hydrogen, 99.99% are in the form of protium (1H) and 0.01% are deuterium (2H or D). Carbon-13 (13C) and deuterium (2H or D) are rare isotopes in methane molecules. The abundance of the clumped isotopes provides information independent from the traditional carbon or hydrogen isotope composition of methane molecules.

Photosynthesis converts carbon dioxide to carbohydrates via several metabolic pathways that provide energy to an organism and preferentially react with certain stable isotopes of carbon. The selective enrichment of one stable isotope over another creates distinct isotopic fractionations that can be measured and correlated among oxygenic phototrophs. The degree of carbon isotope fractionation is influenced by several factors, including the metabolism, anatomy, growth rate, and environmental conditions of the organism. Understanding these variations in carbon fractionation across species is useful for biogeochemical studies, including the reconstruction of paleoecology, plant evolution, and the characterization of food chains.
In stable isotope geochemistry, the Urey–Bigeleisen–Mayer equation, also known as the Bigeleisen–Mayer equation or the Urey model, is a model describing the approximate equilibrium isotope fractionation in an isotope exchange reaction. While the equation itself can be written in numerous forms, it is generally presented as a ratio of partition functions of the isotopic molecules involved in a given reaction. The Urey–Bigeleisen–Mayer equation is widely applied in the fields of quantum chemistry and geochemistry and is often modified or paired with other quantum chemical modelling methods to improve accuracy and precision and reduce the computational cost of calculations.
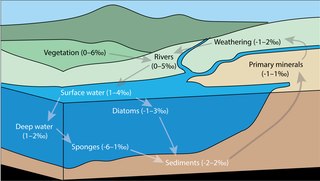
Silicon isotope biogeochemistry is the study of environmental processes using the relative abundance of Si isotopes. As the relative abundance of Si stable isotopes varies among different natural materials, the differences in abundance can be used to trace the source of Si, and to study biological, geological, and chemical processes. The study of stable isotope biogeochemistry of Si aims to quantify the different Si fluxes in the global biogeochemical silicon cycle, to understand the role of biogenic silica within the global Si cycle, and to investigate the applications and limitations of the sedimentary Si record as an environmental and palaeoceanographic proxy.
References
- 1 2 Eiler, J.M. (2007). ""Clumped-isotope" geochemistry—The study of naturally-occurring, multiply-substituted isotopologues". Earth and Planetary Science Letters. 262 (3–4): 309–327. Bibcode:2007E&PSL.262..309E. doi:10.1016/j.epsl.2007.08.020.
- ↑ Lea, D.W. (2014). "8.14 - Elemental and Isotopic Proxies of Past Ocean Temperatures". In Holland, H.D.; Turekian, K.K. (eds.). Treatise on Geochemistry, Second Edition. Vol. 8. Oxford: Elsevier. pp. 373–397. doi:10.1016/B978-0-08-095975-7.00614-8. ISBN 9780080983004.
- ↑ Ghosh, P.; Adkins, J.; Affek, H.; et al. (2006). "13C-18O bonds in carbonate minerals: A new kind of paleothermometer". Geochimica et Cosmochimica Acta. 70 (6): 1439–1456. Bibcode:2006GeCoA..70.1439G. doi:10.1016/j.gca.2005.11.014.
- ↑ Ghosh, P.; Eiler, J.; Campana, S.E.; Feeney, R.F. (2007). "Calibration of the carbonate 'clumped isotope' paleothermometer for otoliths". Geochimica et Cosmochimica Acta. 71 (11): 2736–2744. Bibcode:2007GeCoA..71.2736G. doi:10.1016/j.gca.2007.03.015.
- ↑ Tripati, A.K.; Eagle, R.A.; Thiagarajan, N.; et al. (2010). "13C-18O isotope signatures and 'clumped isotope' thermometry in foraminifera and coccoliths". Geochimica et Cosmochimica Acta. 74 (20): 5697–5717. Bibcode:2010GeCoA..74.5697T. doi:10.1016/j.gca.2010.07.006.
- ↑ Affek, Hagit (2012). "Clumped isotope paleothermometry: Principles, applications, and challenges". Reconstructing Earth's Deep-Time Climate—The State of the Art in 2012, Paleontological Society Short Course, November 3, 2012. 8: 101–114.
- ↑ Urey, H.C. (1947). "The thermodynamic properties of isotopic substances". J. Chem. Soc. London: 562–581. doi:10.1039/JR9470000562. PMID 20249764.
- ↑ Bigeleisen, J.; Mayer, M.G. (1947). "Calculation of equilibrium constants for isotopic exchange reactions". J. Chem. Phys. 15 (5): 261–267. Bibcode:1947JChPh..15..261B. doi:10.1063/1.1746492. hdl: 2027/mdp.39015074123996 .
- ↑ Wang, Z., Schauble, E.A., Eiler, J.M., 2004. Equilibrium thermodynamics of multiply substituted isotopologues of molecular gases. Geochim. Cosmochim. Acta 68, 4779–4797.
- ↑ Chappell, J., Shackleton, N.J., 1986. Oxygen isotopes and sea level. Nature 324, 137–140.
- ↑ C. Waelbroeck, L. Labeyrie, E. Michel, et al., (2002) Sea-level and deep water temperature changes derived from benthic foraminifera isotopic records. Quaternary Science Reviews. 21: 295-305
- ↑ McCrea, J.M., 1950. On the isotopic chemistry of carbonates and a paleotemperature scale. J. Chem. Phys. 18, 849–857.
- ↑ Swart, P.K., Burns, S.J., Leder, J.J., 1991. Fractionation of the stable isotopes of oxygen and carbon in carbon dioxide during the reaction of calcite with phosphoric acid as a function of temperature and technique. Chem. Geol. (Isot. Geosci. Sec.) 86, 89–96.
- 1 2 Eiler, John M. (2011-12-01). "Paleoclimate reconstruction using carbonate clumped isotope thermometry". Quaternary Science Reviews. 30 (25–26): 3575–3588. Bibcode:2011QSRv...30.3575E. doi:10.1016/j.quascirev.2011.09.001. ISSN 0277-3791.
- ↑ Wacker, Ulrike; Rutz, Tanja; Löffler, Niklas; Conrad, Anika C.; Tütken, Thomas; Böttcher, Michael E.; Fiebig, Jens (December 2016). "Clumped isotope thermometry of carbonate-bearing apatite: Revised sample pre-treatment, acid digestion, and temperature calibration". Chemical Geology. 443: 97–110. Bibcode:2016ChGeo.443...97W. doi:10.1016/j.chemgeo.2016.09.009.
- 1 2 3 Löffler, N.; Fiebig, J.; Mulch, A.; Tütken, T.; Schmidt, B.C.; Bajnai, D.; Conrad, A.C.; Wacker, U.; Böttcher, M.E. (May 2019). "Refining the temperature dependence of the oxygen and clumped isotopic compositions of structurally bound carbonate in apatite". Geochimica et Cosmochimica Acta. 253: 19–38. Bibcode:2019GeCoA.253...19L. doi:10.1016/j.gca.2019.03.002. S2CID 107992832.
- ↑ Eiler, J.M.; Schauble, E. (2004). "18O13C16O in earth's atmosphere" (PDF). Geochim. Cosmochim. Acta. 68 (23): 4767–4777. Bibcode:2004GeCoA..68.4767E. doi:10.1016/j.gca.2004.05.035.
- 1 2 Huntington, K. W.; Budd, D. A.; Wernicke, B. P.; Eiler, J. M. (2011-09-01). "Use of Clumped-Isotope Thermometry To Constrain the Crystallization Temperature of Diagenetic Calcite". Journal of Sedimentary Research. 81 (9): 656–669. Bibcode:2011JSedR..81..656H. doi:10.2110/jsr.2011.51. ISSN 1527-1404.
- ↑ Came, Rosemarie E.; Eiler, John M.; Veizer, Ján; Azmy, Karem; Brand, Uwe; Weidman, Christopher R. (September 2007). "Coupling of surface temperatures and atmospheric CO2 concentrations during the Palaeozoic era" (PDF). Nature. 449 (7159): 198–201. Bibcode:2007Natur.449..198C. doi:10.1038/nature06085. ISSN 1476-4687. PMID 17851520. S2CID 4388925.
- ↑ Finnegan, S.; Bergmann, K. D.; Eiler, J.; Jones, D. S.; Fike, D. A.; Eisenman, I. L.; Hughes, N.; Tripati, A. K.; Fischer, W. W. (2010-12-01). "Constraints on the duration and magnitude of Late Ordovician-Early Silurian glaciation and its relationship to the Late Ordovician mass extinction from carbonate clumped isotope paleothermometry". AGU Fall Meeting Abstracts. 54: B54B–04. Bibcode:2010AGUFM.B54B..04F.
- ↑ Santi, L. M.; Arnold, A. J.; Ibarra, D. E.; Whicker, C. A.; Mering, J. A.; Lomarda, R. B.; Lora, J. M.; Tripati, A. (2020-11-01). "Clumped isotope constraints on changes in latest Pleistocene hydroclimate in the northwestern Great Basin: Lake Surprise, California". GSA Bulletin. 132 (11–12): 2669–2683. Bibcode:2020GSAB..132.2669S. doi: 10.1130/B35484.1 . ISSN 0016-7606.
- ↑ Mering, John Arthur (2015). New constraints on water temperature at Lake Bonneville from carbonate clumped isotopes (Master of Science in Geochemistry thesis). University of California, Los Angeles. ProQuest 1707901550.
- 1 2 Ghosh, Prosenjit; Garzione, Carmala N.; Eiler, John M. (2006-01-27). "Rapid Uplift of the Altiplano Revealed Through 13C-18O Bonds in Paleosol Carbonates". Science. 311 (5760): 511–515. Bibcode:2006Sci...311..511G. doi:10.1126/science.1119365. ISSN 0036-8075. PMID 16439658. S2CID 129743191.
- 1 2 Huntington, K. W.; Wernicke, B. P.; Eiler, J. M. (2010-06-01). "Influence of climate change and uplift on Colorado Plateau paleotemperatures from carbonate clumped isotope thermometry" (PDF). Tectonics. 29 (3): TC3005. Bibcode:2010Tecto..29.3005H. doi: 10.1029/2009TC002449 . ISSN 1944-9194.
- ↑ Quade, Jay; Breecker, Daniel O.; Daëron, Mathieu; Eiler, John (2011-02-01). "The paleoaltimetry of Tibet: An isotopic perspective". American Journal of Science. 311 (2): 77–115. Bibcode:2011AmJS..311...77Q. doi: 10.2475/02.2011.01 . ISSN 0002-9599. S2CID 129751114.
- ↑ Hren, Michael T.; Sheldon, Nathan D. (2012-07-01). "Temporal variations in lake water temperature: Paleoenvironmental implications of lake carbonate δ18O and temperature records". Earth and Planetary Science Letters. 337–338: 77–84. Bibcode:2012E&PSL.337...77H. doi:10.1016/j.epsl.2012.05.019. ISSN 0012-821X.
- ↑ Garzione, Carmala N.; Hoke, Gregory D.; Libarkin, Julie C.; Withers, Saunia; MacFadden, Bruce; Eiler, John; Ghosh, Prosenjit; Mulch, Andreas (2008-06-06). "Rise of the Andes". Science. 320 (5881): 1304–1307. Bibcode:2008Sci...320.1304G. doi:10.1126/science.1148615. ISSN 0036-8075. PMID 18535236. S2CID 21288149.
- ↑ Eiler, John M.; Schauble, Edwin (2004-12-01). "18O13C16O in Earth's atmosphere". Geochimica et Cosmochimica Acta. 68 (23): 4767–4777. Bibcode:2004GeCoA..68.4767E. doi:10.1016/j.gca.2004.05.035. ISSN 0016-7037.
- ↑ Laskar, Amzad H.; Mahata, Sasadhar; Liang, Mao-Chang (2016). "Identification of Anthropogenic CO2 Using Triple Oxygen and Clumped Isotopes". Environmental Science & Technology. 50 (21): 11806–11814. Bibcode:2016EnST...5011806L. doi:10.1021/acs.est.6b02989. PMID 27690222.
- 1 2 Eagle, Robert A.; Schauble, Edwin A.; Tripati, Aradhna K.; Tütken, Thomas; Hulbert, Richard C.; Eiler, John M. (2010-06-08). "Body temperatures of modern and extinct vertebrates from 13C-18O bond abundances in bioapatite". Proceedings of the National Academy of Sciences. 107 (23): 10377–10382. Bibcode:2010PNAS..10710377E. doi: 10.1073/pnas.0911115107 . ISSN 0027-8424. PMC 2890843 . PMID 20498092.
- ↑ Eagle, Robert A.; Tütken, Thomas; Martin, Taylor S.; Tripati, Aradhna K.; Fricke, Henry C.; Connely, Melissa; Cifelli, Richard L.; Eiler, John M. (2011-07-22). "Dinosaur Body Temperatures Determined from Isotopic (13C-18O) Ordering in Fossil Biominerals". Science. 333 (6041): 443–445. Bibcode:2011Sci...333..443E. doi:10.1126/science.1206196. ISSN 0036-8075. PMID 21700837. S2CID 206534244.
- ↑ Passey, Benjamin H.; Henkes, Gregory A. (2012-10-15). "Carbonate clumped isotope bond reordering and geospeedometry". Earth and Planetary Science Letters. 351–352: 223–236. Bibcode:2012E&PSL.351..223P. doi:10.1016/j.epsl.2012.07.021. ISSN 0012-821X.
- ↑ Guo, Weifu; Eiler, John M. (2007-11-15). "Temperatures of aqueous alteration and evidence for methane generation on the parent bodies of the CM chondrites". Geochimica et Cosmochimica Acta. 71 (22): 5565–5575. Bibcode:2007GeCoA..71.5565G. CiteSeerX 10.1.1.425.1442 . doi:10.1016/j.gca.2007.07.029. ISSN 0016-7037.
- ↑ Halevy, Itay; Fischer, Woodward W.; Eiler, John M. (2011-10-11). "Carbonates in the Martian meteorite Allan Hills 84001 formed at 18 ± 4 °C in a near-surface aqueous environment". Proceedings of the National Academy of Sciences. 108 (41): 16895–16899. Bibcode:2011PNAS..10816895H. doi: 10.1073/pnas.1109444108 . ISSN 0027-8424. PMC 3193235 . PMID 21969543.
- 1 2 Mering, John; Barker, Shaun; Huntington, Katherine; Simmons, Stuart; Dipple, Gregory; Andrew, Benjamin; Schauer, Andrew (2018-12-01). "Taking the Temperature of Hydrothermal Ore Deposits Using Clumped Isotope Thermometry". Economic Geology. 113 (8): 1671–1678. Bibcode:2018EcGeo.113.1671M. doi:10.5382/econgeo.2018.4608. ISSN 0361-0128. S2CID 135261236.
- ↑ Kirk, Ruth; Marca, Alina; Myhill, Daniel J.; Dennis, Paul F. (2018-01-01). "Clumped isotope evidence for episodic, rapid flow of fluids in a mineralized fault system in the Peak District, UK". Journal of the Geological Society. 176 (3): jgs2016–117. doi:10.1144/jgs2016-117. ISSN 0016-7649. S2CID 133785532.
- ↑ Kluge, Tobias; John, Cédric M.; Boch, Ronny; Kele, Sándor (2018). "Assessment of Factors Controlling Clumped Isotopes and δ18O Values of Hydrothermal Vent Calcites". Geochemistry, Geophysics, Geosystems. 19 (6): 1844–1858. Bibcode:2018GGG....19.1844K. doi:10.1029/2017GC006969. hdl: 10044/1/63564 . ISSN 1525-2027. S2CID 135107115.
- ↑ Kele, Sándor; Breitenbach, Sebastian F.M.; Capezzuoli, Enrico; Meckler, A. Nele; Ziegler, Martin; Millan, Isabel M.; Kluge, Tobias; Deák, József; Hanselmann, Kurt; John, Cédric M.; Yan, Hao; Liu, Zaihua; Bernasconi, Stefano M. (2015). "Temperature dependence of oxygen- and clumped isotope fractionation in carbonates: A study of travertines and tufas in the 6–95°C temperature range" (PDF). Geochimica et Cosmochimica Acta. 168: 172–192. Bibcode:2015GeCoA.168..172K. doi:10.1016/j.gca.2015.06.032.