
Methylecgonine cinnamate is a natural tropane alkaloid found within the coca plant. Its more common name, cinnamoylcocaine, reflects its close structural similarity to cocaine. It is pharmacologically inactive, but some studies funded by anti-drug agencies imply that it is active when smoked. Furthermore, the discovery of differing impurity products yielding methylecgonine cinnamate in confiscated cocaine have led enforcing agencies to postulate that illicit manufacturers have changed their oxidation procedures when refining cocaine from a crude form. Methylecgonine cinnamate can dimerize to the truxillic acid derivative truxilline. It is notable that methylecgonine cinnamate is given in patents of active cocaine analogue structures.

Cocaethylene (ethylbenzoylecgonine) is the ethyl ester of benzoylecgonine. It is structurally similar to cocaine, which is the methyl ester of benzoylecgonine. Cocaethylene is formed by the liver when cocaine and ethanol coexist in the blood. In 1885, cocaethylene was first synthesized, and in 1979, cocaethylene's side effects were discovered.

(–)-2-β-Carbomethoxy-3-β-(4-fluorophenyl)tropane is a stimulant drug used in scientific research. CFT is a phenyltropane based dopamine reuptake inhibitor and is structurally derived from cocaine. It is around 3-10x more potent than cocaine and lasts around 7 times longer based on animal studies. While the naphthalenedisulfonate salt is the most commonly used form in scientific research due to its high solubility in water, the free base and hydrochloride salts are known compounds and can also be produced. The tartrate is another salt form that is reported.

Phenyltropanes (PTs) were originally developed to reduce cocaine addiction and dependency. In general these compounds act as inhibitors of the plasmalemmal monoamine reuptake transporters. Although RTI holds a strong position in this field, they are not the only researchers that have prepared these analogues. This research has spanned beyond the last couple decades, and has picked up its pace in recent times, creating numerous phenyltropanes as research into cocaine analogues garners interest to treat addiction.

Methylecgonidine is a chemical intermediate derived from ecgonine or cocaine.

(+)-CPCA is a stimulant drug similar in structure to pethidine and to RTI-31, but nocaine is lacking the two-carbon bridge of RTI-31's tropane skeleton. This compound was first developed as a substitute agent for cocaine.

Troparil is a stimulant drug used in scientific research. Troparil is a phenyltropane-based dopamine reuptake inhibitor (DRI) that is derived from methylecgonidine. Troparil is a few times more potent than cocaine as a dopamine reuptake inhibitor, but is less potent as a serotonin reuptake inhibitor, and has a duration spanning a few times longer, since the phenyl ring is directly connected to the tropane ring through a non-hydrolyzable carbon-carbon bond. The lack of an ester linkage removes the local anesthetic action from the drug, so troparil is a pure stimulant. This change in activity also makes troparil slightly less cardiotoxic than cocaine. The most commonly used form of troparil is the tartrate salt, but the hydrochloride and naphthalenedisulfonate salts are also available, as well as the free base.

Dimethocaine, also known as DMC or larocaine, is a compound with a stimulatory effect. This effect resembles that of cocaine, although dimethocaine appears to be less potent. Just like cocaine, dimethocaine is addictive due to its stimulation of the reward pathway in the brain. However, dimethocaine is a legal cocaine replacement in some countries and is even listed by the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) under the category “synthetic cocaine derivatives”. The structure of dimethocaine, being a 4-aminobenzoic acid ester, resembles that of procaine. It is found as a white powder at room temperature.
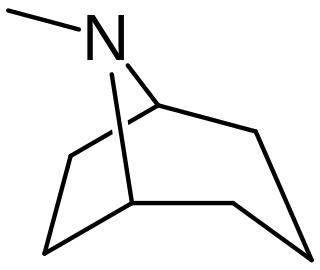
Tropane alkaloids are a class of bicyclic [3.2.1] alkaloids and secondary metabolites that contain a tropane ring in their chemical structure. Tropane alkaloids occur naturally in many members of the plant family Solanaceae. Certain tropane alkaloids such as cocaine and scopolamine are notorious for their psychoactive effects, related usage and cultural associations. Particular tropane alkaloids such as these have pharmacological properties and can act as anticholinergics or stimulants.

Dichloropane ((−)-2β-Carbomethoxy-3β-(3,4-dichlorophenyl)tropane, RTI-111, O-401) is a stimulant of the phenyltropane class that acts as a serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI) with IC50 values of 3.13, 0.79 and 18 nM, respectively. In animal studies, dichloropane had a slower onset and longer duration of action compared to cocaine.

3β-(p-Fluorobenzoyloxy)tropane, is a tropane derivative drug which acts as a local anaesthetic, having around 30% the stimulant potency of cocaine but around the same potency as a local anaesthetic. It has been investigated as a potential radiolabelled agent for studying receptor binding, but was not adopted for this application. The main application for fluorotropacocaine, however, has been as a designer drug analogue of cocaine, first detected by the EMCDDA in 2008, and subsequently sold as an ingredient of various "bath salt" powder products, usually mixed in combination with other stimulant drugs such as caffeine, dimethocaine, desoxypipradrol or substituted cathinone derivatives.

RTI-126 is a phenyltropane derivative which acts as a potent monoamine reuptake inhibitor and stimulant drug, and has been sold as a designer drug. It is around 5 times more potent than cocaine at inhibiting monoamine reuptake in vitro, but is relatively unselective. It binds to all three monoamine transporters, although still with some selectivity for the dopamine transporter. RTI-126 has a fast onset of effects and short duration of action, and its pharmacological profile in animals is among the closest to cocaine itself out of all the drugs in the RTI series. Its main application in scientific research has been in studies investigating the influence of pharmacokinetics on the abuse potential of stimulant drugs, with its rapid entry into the brain thought to be a key factor in producing its high propensity for development of dependence in animals.
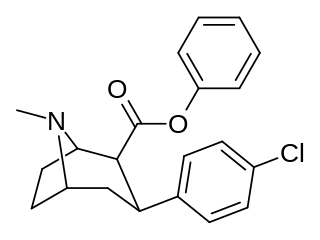
RTI(-4229)-113 is a stimulant drug which acts as a potent and fully selective dopamine reuptake inhibitor (DRI). It has been suggested as a possible substitute drug for the treatment of cocaine addiction. "RTI-113 has properties that make it an ideal medication for cocaine abusers, such as an equivalent efficacy, a higher potency, and a longer duration of action as compared to cocaine." Replacing the methyl ester in RTI-31 with a phenyl ester makes the resultant RTI-113 fully DAT specific. RTI-113 is a particularly relevant phenyltropane cocaine analog that has been tested on squirrel monkeys. RTI-113 has also been tested against cocaine in self-administration studies for DAT occupancy by PET on awake rhesus monkeys. The efficacy of cocaine analogs to elicit self-administration is closely related to the rate at which they are administered. Slower onset of action analogs are less likely to function as positive reinforcers than analogues that have a faster rate of onset.

(–)-2β-Carbomethoxy-3β-(4-tolyl)tropane is a phenyltropane-based cocaine analogue that has similar properties in vitro to related drugs such as RTI-31.

Salicylmethylecgonine, (2′-Hydroxycocaine) is a tropane derivative drug which is both a synthetic analogue and a possible active metabolite of cocaine. Its potency in vitro is around 10x that of cocaine, although it is only around three times more potent than cocaine when administered to mice Note however that the compound 2′-Acetoxycocaine would act as a prodrug to Salicylmethylecgonine in humans, and has a more efficient partition coefficient which would act as a delivery system and would circumvent this reason for a drop in potency. Salicylmethylecgonine also shows increased behavioral stimulation compared to cocaine similar to the phenyltropanes. The hydroxy branch renders the molecule a QSAR of a 10-fold increase over cocaine in its binding potency for the dopamine transporter & a 52-fold enhanced affinity for the norepinephrine transporter. It also has a reduced selectivity for the serotonin transporter though only due to its greater increase at NET binding; its SERT affinity being 4-fold increased compared to cocaine. However, in overall binding affinity it displaces ligands better across the board than cocaine in all monoamine categories.

RTI-229, also known as (–)-3β-(4-iodophenyl)tropane-2β-pyrrolidine carboxamide and RTI-4229-229, is a potent and long-lasting stimulant drug which was developed in the 1990s as part of a large group of related analogues from the phenyltropane family. With the combination of two potent dopamine transporter (DAT) binding motifs attached to the tropane ring, the p-iodophenyl group at the 3β-position and a pyrrolidine carboxamide at 2β, RTI-229 has extremely high selectivity for the dopamine transporter and is one of the most DAT-selective compounds in the RTI series.
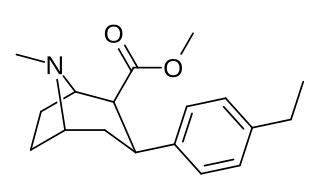
RTI-83 is a phenyltropane derivative which represents a rare example of an SDRI or serotonin-dopamine reuptake inhibitor, a drug which inhibits the reuptake of the neurotransmitters serotonin and dopamine, while having little or no effect on the reuptake of the related neurotransmitter noradrenaline. With a binding affinity (Ki) of 55 nM at DAT and 28.4 nM at SERT but only 4030 nM at NET, RTI-83 has reasonable selectivity for DAT/SERT over NET
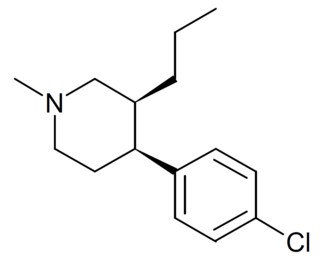
1-Methyl-3-propyl-4-(p-chlorophenyl)piperidine is a drug developed by a team led by Alan Kozikowski, which acts as a potent dopamine reuptake inhibitor, and was developed as a potential therapeutic agent for the treatment of cocaine addiction. As with related compounds such as nocaine, it is a structurally simplified derivative of related phenyltropane compounds. Its activity at the serotonin and noradrenaline transporters has not been published, though most related 4-phenylpiperidine derivatives are relatively selective for inhibiting dopamine reuptake over the other monoamine neurotransmitters. While several of its isomers are active, the (3S,4S)-enantiomer is by far the most potent. The rearranged structural isomer 2-[1-(4-chlorophenyl)butyl]piperidine is also a potent inhibitor of dopamine reuptake.


















