
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen, sulfur and, more rarely, other elements such as chlorine, bromine, and phosphorus.

Cocaine is a stimulant drug obtained from the leaves of two Coca species native to South America, Erythroxylum coca and Erythroxylum novogranatense. After extraction from coca leaves and further processing into cocaine hydrochloride, the drug may be snorted, heated until sublimated and then inhaled, or dissolved and injected into a vein. Cocaine stimulates the reward pathway in the brain. Mental effects may include an intense feeling of happiness, sexual arousal, loss of contact with reality, or agitation. Physical effects may include a fast heart rate, sweating, and dilated pupils. High doses can result in high blood pressure or high body temperature. Effects begin within seconds to minutes of use and last between five and ninety minutes. As cocaine also has numbing and blood vessel constriction properties, it is occasionally used during surgery on the throat or inside of the nose to control pain, bleeding, and vocal cord spasm.

Coca is any of the four cultivated plants in the family Erythroxylaceae, native to western South America. Coca is known worldwide for its psychoactive alkaloid, cocaine.
Clandestine chemistry is chemistry carried out in secret, and particularly in illegal drug laboratories. Larger labs are usually run by gangs or organized crime intending to produce for distribution on the black market. Smaller labs can be run by individual chemists working clandestinely in order to synthesize smaller amounts of controlled substances or simply out of a hobbyist interest in chemistry, often because of the difficulty in ascertaining the purity of other, illegally synthesized drugs obtained on the black market. The term clandestine lab is generally used in any situation involving the production of illicit compounds, regardless of whether the facilities being used qualify as a true laboratory.

Phytochemistry is the study of phytochemicals, which are chemicals derived from plants. Phytochemists strive to describe the structures of the large number of secondary metabolites found in plants, the functions of these compounds in human and plant biology, and the biosynthesis of these compounds. Plants synthesize phytochemicals for many reasons, including to protect themselves against insect attacks and plant diseases. The compounds found in plants are of many kinds, but most can be grouped into four major biosynthetic classes: alkaloids, phenylpropanoids, polyketides, and terpenoids.

Tropinone is an alkaloid, famously synthesised in 1917 by Robert Robinson as a synthetic precursor to atropine, a scarce commodity during World War I. Tropinone and the alkaloids cocaine and atropine all share the same tropane core structure. Its corresponding conjugate acid at pH 7.3 major species is known as tropiniumone.

Ibogaine is a naturally occurring psychoactive substance found in plants in the family Apocynaceae such as Tabernanthe iboga, Voacanga africana, and Tabernaemontana undulata. It is a psychedelic with dissociative properties.
Coca paste is a crude extract of the coca leaf which contains 40% to 91% cocaine freebase along with companion coca alkaloids and varying quantities of benzoic acid, methanol, and kerosene. In South America, coca paste, also known as cocaine base and, therefore, often confused with cocaine sulfate in North America, is relatively inexpensive and is widely used by low-income populations. The coca paste is smoked in tobacco or cannabis cigarettes and use has become widespread in several Latin American countries. Traditionally, coca paste has been relatively abundant in South American countries such as Colombia where it is processed into cocaine hydrochloride for distribution to the rest of the world. The caustic reactions associated with the local application of coca paste prevents its use by oral, intranasal, mucosal, intramuscular, intravenous or subcutaneous routes. Coca paste can only be smoked when combined with a combustible material such as tobacco or cannabis.

Triphenylphosphine oxide (often abbreviated TPPO) is the organophosphorus compound with the formula OP(C6H5)3, also written as Ph3PO or PPh3O (Ph = C6H5). This colourless crystalline compound is a common but potentially useful waste product in reactions involving triphenylphosphine. It is a popular reagent to induce the crystallizing of chemical compounds.

(+)-CPCA is a stimulant drug similar in structure to pethidine and to RTI-31, but nocaine is lacking the two-carbon bridge of RTI-31's tropane skeleton. This compound was first developed as a substitute agent for cocaine.

Troparil is a stimulant drug used in scientific research. Troparil is a phenyltropane-based dopamine reuptake inhibitor (DRI) that is derived from methylecgonidine. Troparil is a few times more potent than cocaine as a dopamine reuptake inhibitor, but is less potent as a serotonin reuptake inhibitor, and has a duration spanning a few times longer, since the phenyl ring is directly connected to the tropane ring through a non-hydrolyzable carbon-carbon bond. The lack of an ester linkage removes the local anesthetic action from the drug, so troparil is a pure stimulant. This change in activity also makes troparil slightly less cardiotoxic than cocaine. The most commonly used form of troparil is the tartrate salt, but the hydrochloride and naphthalenedisulfonate salts are also available, as well as the free base.
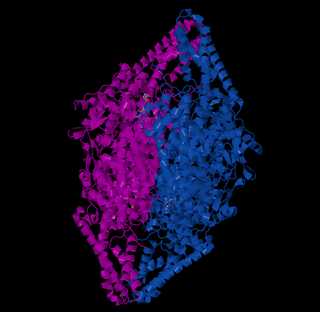
The enzyme phenylalanine ammonia lyase (EC 4.3.1.24) catalyzes the conversion of L-phenylalanine to ammonia and trans-cinnamic acid.:
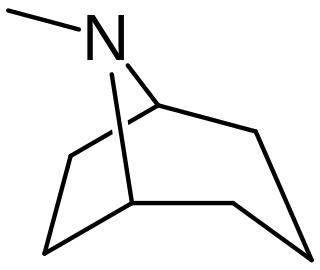
Tropane alkaloids are a class of bicyclic [3.2.1] alkaloids and secondary metabolites that contain a tropane ring in their chemical structure. Tropane alkaloids occur naturally in many members of the plant family Solanaceae. Certain tropane alkaloids such as cocaine and scopolamine are notorious for their psychoactive effects, related usage and cultural associations. Particular tropane alkaloids such as these have pharmacological properties and can act as anticholinergics or stimulants.

LR-5182 is a stimulant drug which acts as a norepinephrine–dopamine reuptake inhibitor, structurally related to the better known drug fencamfamine. It was developed by the pharmaceutical company Eli Lilly in the 1970s, and researched for potential use as an antidepressant, although never marketed. LR-5182 has two stereoisomers, both of which are active, although one isomer blocks reuptake of only dopamine and noradrenaline, while the other blocks reuptake of serotonin as well.

The biosynthesis of cocaine has long attracted the attention of biochemists and organic chemists. This interest is partly motivated by the strong physiological effects of cocaine, but a further incentive was the unusual bicyclic structure of the molecule. The biosynthesis can be viewed as occurring in two phases, one phase leading to the N-methylpyrrolinium ring, which is preserved in the final product. The second phase incorporates a C4 unit with formation of the bicyclic tropane core.

Salicylmethylecgonine, (2′-Hydroxycocaine) is a tropane derivative drug which is both a synthetic analogue and a possible active metabolite of cocaine. Its potency in vitro is around 10x that of cocaine, although it is only around three times more potent than cocaine when administered to mice Note however that the compound 2′-Acetoxycocaine would act as a prodrug to Salicylmethylecgonine in humans, and has a more efficient partition coefficient which would act as a delivery system and would circumvent this reason for a drop in potency. Salicylmethylecgonine also shows increased behavioral stimulation compared to cocaine similar to the phenyltropanes. The hydroxy branch renders the molecule a QSAR of a 10-fold increase over cocaine in its binding potency for the dopamine transporter & a 52-fold enhanced affinity for the norepinephrine transporter. It also has a reduced selectivity for the serotonin transporter though only due to its greater increase at NET binding; its SERT affinity being 4-fold increased compared to cocaine. However, in overall binding affinity it displaces ligands better across the board than cocaine in all monoamine categories.
Coca alkaloids are the alkaloids found in the coca plant, Erythroxylum coca. They are predominantly of either the pyrrolidine or the tropane types.

Methylecgonine is a prominent tropane alkaloid found in coca leaves. It is metabolite of cocaine, and maybe is used as precursor for it. It also occurs as minor alkaloid in roots of many Datura species such as Datura stramonium and Datura innoxia.















