Sodium laureth sulfate (SLES), an accepted contraction of sodium lauryl ether sulfate (SLES), also called sodium alkylethersulfate, is an anionic detergent and surfactant found in many personal care products and for industrial uses. SLES is an inexpensive and very effective foaming agent. SLES, sodium lauryl sulfate (SLS), ammonium lauryl sulfate (ALS), and sodium pareth sulfate are surfactants that are used in many cosmetic products for their cleaning and emulsifying properties. It is derived from palm kernel oil or coconut oil. In herbicides, it is used as a surfactant to improve absorption of the herbicidal chemicals and reduces time the product takes to be rainfast, when enough of the herbicidal agent will be absorbed.

A triglyceride is an ester derived from glycerol and three fatty acids. Triglycerides are the main constituents of body fat in humans and other vertebrates, as well as vegetable fat. They are also present in the blood to enable the bidirectional transference of adipose fat and blood glucose from the liver, and are a major component of human skin oils.

Surfactants are chemical compounds that decrease the surface tension between two liquids, between a gas and a liquid, or interfacial tension between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, foaming agents, or dispersants. The word "surfactant" is a blend of surface-active agent, coined c. 1950.

Coconut oil is an edible oil derived from the wick, meat, and milk of the coconut palm fruit. Coconut oil is a white solid fat; in warmer climates during the summer months it is a clear thin liquid oil, melting at warmer room temperatures of around 25 °C (78 °F). Unrefined varieties have a distinct coconut aroma. It is used as a food oil, and in industrial applications for cosmetics and detergent production. Due to its high levels of saturated fat, numerous health authorities recommend limiting its consumption as a food.
Lactones are cyclic carboxylic esters, containing a 1-oxacycloalkan-2-one structure, or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring.
Erucic acid is a monounsaturated omega-9 fatty acid, denoted 22:1ω9. It has the chemical formula CH3(CH2)7CH=CH(CH2)11COOH. It is prevalent in wallflower seed and other plants in the family Brassicaceae, with a reported content of 20 to 54% in high erucic acid rapeseed oil and 42% in mustard oil. Erucic acid is also known as cis-13-docosenoic acid and the trans isomer is known as brassidic acid.
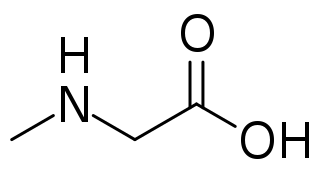
Sarcosine, also known as N-methylglycine, or monomethylglycine, is a monopeptide with the formula CH3N(H)CH2CO2H. It exists at neutral pH as the zwitterion CH3N+(H)2CH2CO2−, which can be obtained as a white, water-soluble powder. Like some amino acids, sarcosine converts to a cation at low pH and an anion at high pH, with the respective formulas CH3N+(H)2CH2CO2H and CH3N(H)CH2CO2−. Sarcosine is a close relative of glycine, with a secondary amine in place of the primary amine.

Cocamide DEA, or cocamide diethanolamine, is a diethanolamide made by reacting the mixture of fatty acids from coconut oils with diethanolamine. It is a viscous liquid and is used as a foaming agent in bath products like shampoos and hand soaps, and in cosmetics as an emulsifying agent. See cocamide for the discussion of the lengths of carbon chains in the molecules in the mixture. The chemical formula of individual components is CH3(CH2)nC(=O)N(CH2CH2OH)2, where n typically ranges from 8 to 18.

In organic chemistry, sulfonic acid refers to a member of the class of organosulfur compounds with the general formula R−S(=O)2−OH, where R is an organic alkyl or aryl group and the S(=O)2(OH) group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound is the parent sulfonic acid, HS(=O)2(OH), a tautomer of sulfurous acid, S(=O)(OH)2. Salts or esters of sulfonic acids are called sulfonates.
Fatty alcohols (or long-chain alcohols) are usually high-molecular-weight, straight-chain primary alcohols, but can also range from as few as 4–6 carbons to as many as 22–26, derived from natural fats and oils. The precise chain length varies with the source. Some commercially important fatty alcohols are lauryl, stearyl, and oleyl alcohols. They are colourless oily liquids (for smaller carbon numbers) or waxy solids, although impure samples may appear yellow. Fatty alcohols usually have an even number of carbon atoms and a single alcohol group (–OH) attached to the terminal carbon. Some are unsaturated and some are branched. They are widely used in industry. As with fatty acids, they are often referred to generically by the number of carbon atoms in the molecule, such as "a C12 alcohol", that is an alcohol having 12 carbons, for example dodecanol.
A foaming agent is a material such as a surfactant or a blowing agent that facilitates the formation of foam. A surfactant, when present in small amounts, reduces surface tension of a liquid or increases its colloidal stability by inhibiting coalescence of bubbles. A blowing agent is a gas that forms the gaseous part of the foam.
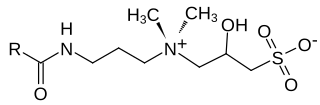
Hydroxysultaines are chemical compounds used in high-foaming shampoos, bath products and shower gels especially in conjunction with ether sulfates and alkyl sulfates. They are also used in industrial applications where high, stable foam is required. Chemically, hydroxysultaines are zwitterionic, typically containing covalently linked positive and negative ions.
An antistatic agent is a compound used for treatment of materials or their surfaces in order to reduce or eliminate buildup of static electricity. Static charge may be generated by the triboelectric effect or by a non-contact process using a high voltage power source. Static charge may be introduced on a surface as part of an in-mold label printing process.
Cocamidopropyl betaine (CAPB) is a mixture of closely related organic compounds derived from coconut oil and dimethylaminopropylamine. CAPB is available as a viscous pale yellow solution and it is used as a surfactant in personal care products. The name reflects that the major part of the molecule, the lauric acid group, is derived from coconut oil. Cocamidopropyl betaine to a significant degree has replaced cocamide DEA.

An amphiphile is a chemical compound possessing both hydrophilic and lipophilic (fat-loving) properties. Such a compound is called amphiphilic or amphipathic. Common amphiphilic substances are soaps, detergents, and lipoproteins. The phospholipid amphiphiles are the major structural component of cell membranes.
1-Tetradecanol, or commonly myristyl alcohol (from Myristica fragrans – the nutmeg plant), is a straight-chain saturated fatty alcohol, with the molecular formula CH3(CH2)12CH2OH. It is a white waxy solid that is practically insoluble in water, soluble in diethyl ether, and slightly soluble in ethanol.

1-Decanol is a straight chain fatty alcohol with ten carbon atoms and the molecular formula C10H21OH. It is a colorless to light yellow viscous liquid that is insoluble in water and has an aromatic odor. The interfacial tension against water at 20 °C is 8.97 mN/m.

Shampoo is a hair care product, typically in the form of a viscous liquid, that is used for cleaning hair. Less commonly, shampoo is available in solid bar format. Shampoo is used by applying it to wet hair, massaging the product into the scalp, and then rinsing it out. Some users may follow a shampooing with the use of hair conditioner.

Sucrose esters or sucrose fatty acid esters are a group of non-naturally occurring surfactants chemically synthesized from the esterification of sucrose and fatty acids. This group of substances is remarkable for the wide range of hydrophilic-lipophilic balance (HLB) that it covers. The polar sucrose moiety serves as a hydrophilic end of the molecule, while the long fatty acid chain serves as a lipophilic end of the molecule. Due to this amphipathic property, sucrose esters act as emulsifiers; i.e., they have the ability to bind both water and oil simultaneously. Depending on the HLB value, some can be used as water-in-oil emulsifiers, and some as oil-in-water emulsifiers. Sucrose esters are used in cosmetics, food preservatives, food additives, and other products. A class of sucrose esters with highly substituted hydroxyl groups, olestra, is also used as a fat replacer in food.
Isethionates are esters of long-chain aliphatic carboxylic acids (C8 – C18) with isethionic acid (2-hydroxyethanesulfonic acid) or salts thereof, such as ammonium isethionate or sodium isethionate. They are also referred to as acyl isethionates or acyloxyethanesulfonates.











