
A triglyceride is an ester derived from glycerol and three fatty acids. Triglycerides are the main constituents of body fat in humans and other vertebrates, as well as vegetable fat. They are also present in the blood to enable the bidirectional transference of adipose fat and blood glucose from the liver, and are a major component of human skin oils.
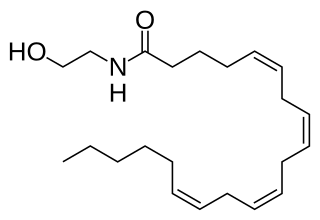
Anandamide (ANA), also known as N-arachidonoylethanolamine (AEA), is a fatty acid neurotransmitter. Anandamide was the first endocannabinoid to be discovered: it participates in the body's endocannabinoid system by binding to cannabinoid receptors, the same receptors that the psychoactive compound THC in cannabis acts on. Anandamide is found in nearly all tissues in a wide range of animals. Anandamide has also been found in plants, including small amounts in chocolate. The name 'anandamide' is taken from the Sanskrit word ananda, which means "joy, bliss, delight", and amide.

Coconut oil is an edible oil derived from the wick, meat, and milk of the coconut palm fruit. Coconut oil is a white solid fat, melting at warmer room temperatures of around 25 °C (78 °F), in warmer climates during the summer months it is a clear thin liquid oil. Unrefined varieties have a distinct coconut aroma. It is used as a food oil, and in industrial applications for cosmetics and detergent production. Due to its high levels of saturated fat, numerous health authorities recommend limiting its consumption as a food.

Stearic acid ( STEER-ik, stee-ARR-ik) is a saturated fatty acid with an 18-carbon chain. The IUPAC name is octadecanoic acid. It is a waxy solid and its chemical formula is C17H35CO2H. Its name comes from the Greek word στέαρ "stéar", which means tallow. The salts and esters of stearic acid are called stearates. As its ester, stearic acid is one of the most common saturated fatty acids found in nature following palmitic acid. The triglyceride derived from three molecules of stearic acid is called stearin.

A drying oil is an oil that hardens to a tough, solid film after a period of exposure to air, at room temperature. The oil hardens through a chemical reaction in which the components crosslink by the action of oxygen. Drying oils are a key component of oil paint and some varnishes. Some commonly used drying oils include linseed oil, tung oil, poppy seed oil, perilla oil, and walnut oil. Their use has declined over the past several decades, as they have been replaced by alkyd resins and other binders.

Ethanolamine (2-aminoethanol, monoethanolamine, ETA, or MEA) is an organic chemical compound with the formula HOCH2CH2NH2 or C2H7NO. The molecule is bifunctional, containing both a primary amine and a primary alcohol. Ethanolamine is a colorless, viscous liquid with an odor reminiscent of ammonia. ETA molecules are a component in the formation of cellular membranes and are thus a molecular building block for life. It was thought to exist only on Earth and on certain asteroids, but in 2021 evidence was found that ETA molecules exist in interstellar space.

N-Methylethanolamine is an alkanolamine with the formula CH3NHCH2CH2OH. It is flammable, corrosive, colorless, viscous liquid. It is an intermediate in the biosynthesis of choline.

Glycerophospholipids or phosphoglycerides are glycerol-based phospholipids. They are the main component of biological membranes.
Cocamide is a mixture of amides manufactured from the fatty acids obtained from coconut oil. As coconut oil contains about 50% of lauric acid, in formulas only the 12-carbon chains tend to be considered. Therefore the formula of cocamide can be written as CH3(CH2)10CONH2, though the number of carbon atoms in the chains varies (it is always even).
Ethylenediamine (abbreviated as en when a ligand) is the organic compound with the formula C2H4(NH2)2. This colorless liquid with an ammonia-like odor is a basic amine. It is a widely used building block in chemical synthesis, with approximately 500,000 tonnes produced in 1998. Ethylenediamine readily reacts with moisture in humid air to produce a corrosive, toxic and irritating mist, to which even short exposures can cause serious damage to health (see safety). Ethylenediamine is the first member of the so-called polyethylene amines.

Diethanolamine, often abbreviated as DEA or DEOA, is an organic compound with the formula HN(CH2CH2OH)2. Pure diethanolamine is a white solid at room temperature, but its tendencies to absorb water and to supercool meaning that it is often encountered as a colorless, viscous liquid. Diethanolamine is polyfunctional, being a secondary amine and a diol. Like other organic amines, diethanolamine acts as a weak base. Reflecting the hydrophilic character of the secondary amine and hydroxyl groups, DEA is soluble in water. Amides prepared from DEA are often also hydrophilic. In 2013, the chemical was classified by the International Agency for Research on Cancer as "possibly carcinogenic to humans" (Group 2B).

Phosphatidylethanolamine (PE) is a class of phospholipids found in biological membranes. They are synthesized by the addition of cytidine diphosphate-ethanolamine to diglycerides, releasing cytidine monophosphate. S-Adenosyl methionine can subsequently methylate the amine of phosphatidylethanolamines to yield phosphatidylcholines. It can mainly be found in the inner (cytoplasmic) leaflet of the lipid bilayer.
Monoethanolamine oleate (properly ethanolammonium oleate) is an antivaricose agent. It is a salt formed by Lowry–Brønsted acid–base reaction between monoethanolamine and oleic acid, is systematically named 2-hydroxyethylammonium (9Z)-octadecenoate, and has a structural formula [CH3(CH2)7CH=CH(CH2)7CO2][H3NCH2CH2OH]. It is injected topically into varicosities to cause sclerosis (closure) of the abnormal vein.
In enzymology, an acyl-[acyl-carrier-protein]-phospholipid O-acyltransferase is an enzyme that catalyzes the chemical reaction
Cleaning agents or hard-surface cleaners are substances used to remove dirt, including dust, stains, bad smells, and clutter on surfaces. Purposes of cleaning agents include health, beauty, removing offensive odor, and avoiding the spread of dirt and contaminants to oneself and others. Some cleaning agents can kill bacteria and clean at the same time. Others, called degreasers, contain organic solvents to help dissolve oils and fats.

An N-acylethanolamine (NAE) is a type of fatty acid amide formed when one of several types of acyl group is linked to the nitrogen atom of ethanolamine. These amides conceptually can be formed from a fatty acid and ethanolamine with the release of a molecule of water, but the known biological synthesis uses a specific phospholipase D to cleave the phospholipid unit from N-acylphosphatidylethanolamines. Another route relies on the transesterification of acyl groups from phosphatidylcholine by an N-acyltransferase (NAT) activity. The suffixes -amine and -amide in these names each refer to the single nitrogen atom of ethanolamine that links the compound together: it is termed "amine" in ethanolamine because it is considered as a free terminal nitrogen in that subunit, while it is termed "amide" when it is considered in association with the adjacent carbonyl group of the acyl subunit. Names for these compounds may be encountered with either "amide" or "amine" varying by author.

Diethanolamides are common ingredients used in cosmetics to act as a foaming agents or as emulsifiers. Chemically, they are amides formed from diethanolamine and carboxylic acids, typically fatty acids.

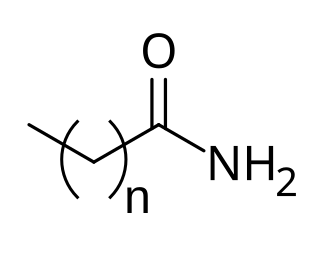
Fatty acid amides (FAAs) are amides formed from a fatty acid and an amine. In nature, many FAAs have ethanolamine as the amine component. Also known as N-acylethanolamines, they contain the functionality RC(O)N(H)CH2CH2OH. A well known example is anandamide. Other fatty acid amides are fatty acid primary amides (FAPAs). They contain the functionality RC(O)NH2). Oleamide is an example of this class of FAPAs.

Ethanolamides are chemical compounds which are amides formed from carboxylic acids and ethanolamine. Some ethanolamides are naturally occurring, such as anandamide, palmitoylethanolamide and prostamides, which play physiological roles as lipid neurotransmitters and autacoids.

Ambadi seed oil is extracted from seeds of the ambadi plant. It is an annual or perennial plant in the family Malvaceae and related to the roselle. It is believed to be native to Africa or Tropical Asia.

















