
In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.
Sodium laureth sulfate (SLES), an accepted contraction of sodium lauryl ether sulfate (SLES), also called sodium alkylethersulfate, is an anionic detergent and surfactant found in many personal care products and for industrial uses. SLES is an inexpensive and very effective foaming agent. SLES, sodium lauryl sulfate (SLS), ammonium lauryl sulfate (ALS), and sodium pareth sulfate are surfactants that are used in many cosmetic products for their cleaning and emulsifying properties. It is derived from palm kernel oil or coconut oil. In herbicides, it is used as a surfactant to improve absorption of the herbicidal chemicals and reduces time the product takes to be rainfast, when enough of the herbicidal agent will be absorbed.
Sodium dodecyl sulfate (SDS) or sodium lauryl sulfate (SLS), sometimes written sodium laurilsulfate, is an organic compound with the formula CH3(CH2)11OSO3Na and structure H3C−(CH2)11−O−S(=O)2−O−Na+. It is an anionic surfactant used in many cleaning and hygiene products. This compound is the sodium salt of the 12-carbon organosulfate. Its hydrocarbon tail combined with a polar "headgroup" give the compound amphiphilic properties that make it useful as a detergent. SDS is also component of mixtures produced from inexpensive coconut and palm oils. SDS is a common component of many domestic cleaning, personal hygiene and cosmetic, pharmaceutical, and food products, as well as of industrial and commercial cleaning and product formulations.

A triglyceride is an ester derived from glycerol and three fatty acids. Triglycerides are the main constituents of body fat in humans and other vertebrates, as well as vegetable fat. They are also present in the blood to enable the bidirectional transference of adipose fat and blood glucose from the liver, and are a major component of human skin oils.

A detergent is a surfactant or a mixture of surfactants with cleansing properties when in dilute solutions. There are a large variety of detergents, a common family being the alkylbenzene sulfonates, which are soap-like compounds that are more soluble in hard water, because the polar sulfonate is less likely than the polar carboxylate to bind to calcium and other ions found in hard water.

Surfactants are chemical compounds that decrease the surface tension or interfacial tension between two liquids, a liquid and a gas, or a liquid and a solid. The word "surfactant" is a blend of surface-active agent, coined c. 1950. As they consist of a water-repellent and a water-attracting part, they enable water and oil to mix; they can form foam and facilitate the detachment of dirt.

Palmitic acid is a fatty acid with a 16-carbon chain. It is the most common saturated fatty acid found in animals, plants and microorganisms. Its chemical formula is CH3(CH2)14COOH, and its C:D ratio is 16:0. It is a major component of palm oil from the fruit of Elaeis guineensis, making up to 44% of total fats. Meats, cheeses, butter, and other dairy products also contain palmitic acid, amounting to 50–60% of total fats.
Ammonium lauryl sulfate (ALS) is the common name for ammonium dodecyl sulfate (CH3(CH2)10CH2OSO3NH4). The anion consists of a nonpolar hydrocarbon chain and a polar sulfate end group. The combination of nonpolar and polar groups confers surfactant properties to the anion: it facilitates dissolution of both polar and non-polar materials. This salt is classified as a sulfate ester. It is made from coconut or palm kernel oil for use primarily in shampoos and body-wash as a foaming agent. Lauryl sulfates are very high-foam surfactants that disrupt the surface tension of water in part by forming micelles at the surface-air interface.
Fatty alcohols (or long-chain alcohols) are usually high-molecular-weight, straight-chain primary alcohols, but can also range from as few as 4–6 carbons to as many as 22–26, derived from natural fats and oils. The precise chain length varies with the source. Some commercially important fatty alcohols are lauryl, stearyl, and oleyl alcohols. They are colourless oily liquids (for smaller carbon numbers) or waxy solids, although impure samples may appear yellow. Fatty alcohols usually have an even number of carbon atoms and a single alcohol group (–OH) attached to the terminal carbon. Some are unsaturated and some are branched. They are widely used in industry. As with fatty acids, they are often referred to generically by the number of carbon atoms in the molecule, such as "a C12 alcohol", that is an alcohol having 12 carbons, for example dodecanol.
Dodecanol, or lauryl alcohol, is an organic compound produced industrially from palm kernel oil or coconut oil. It is a fatty alcohol. Sulfate esters of lauryl alcohol, especially sodium lauryl sulfate, are very widely used as surfactants. Sodium lauryl sulfate and the related dodecanol derivatives ammonium lauryl sulfate and sodium laureth sulfate are all used in shampoos. Dodecanol is tasteless, colorless, and has a floral odor.
A foaming agent is a material such as a surfactant or a blowing agent that facilitates the formation of foam. A surfactant, when present in small amounts, reduces surface tension of a liquid or increases its colloidal stability by inhibiting coalescence of bubbles. A blowing agent is a gas that forms the gaseous part of the foam.
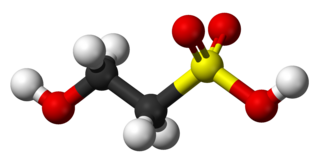
Isethionic acid is an organosulfur compound containing an alkylsulfonic acid located beta to a hydroxy group. Its discovery is generally attributed to Heinrich Gustav Magnus, who prepared it by the action of solid sulfur trioxide on ethanol in 1833. It is a white water-soluble solid used in the manufacture of certain surfactants and in the industrial production of taurine. It is most commonly available in the form of its sodium salt.
Sodium myreth sulfate is a mixture of organic compounds with both detergent and surfactant properties. It is found in many personal care products such as soaps, shampoos, and toothpaste. It is an inexpensive and effective foaming agent. Typical of many detergents, sodium myreth sulfate consists of several closely related compounds. Sometimes the number of ethylene glycol ether units (n) is specified in the name as myreth-n sulfate, for example myreth-2 sulfate.
Cocamidopropyl betaine (CAPB) is a mixture of closely related organic compounds derived from coconut oil and dimethylaminopropylamine. CAPB is available as a viscous pale yellow solution and it is used as a surfactant in personal care products and animal husbandry. The name reflects that the major part of the molecule, the lauric acid group, is derived from coconut oil. Cocamidopropyl betaine to a significant degree has replaced cocamide DEA.
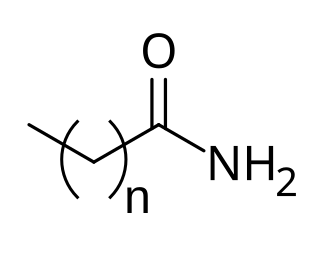
Cocamide is a mixture of amides manufactured from the fatty acids obtained from coconut oil. As coconut oil contains about 50% of lauric acid, in formulas only the 12-carbon chains tend to be considered. Therefore the formula of cocamide can be written as CH3(CH2)10CONH2, though the number of carbon atoms in the chains varies.
Sulfation is the chemical reaction that entails the addition of SO3 group. In principle, many sulfations would involve reactions of sulfur trioxide (SO3). In practice, most sulfations are effected less directly. Regardless of the mechanism, the installation of a sulfate-like group on a substrate leads to substantial changes.
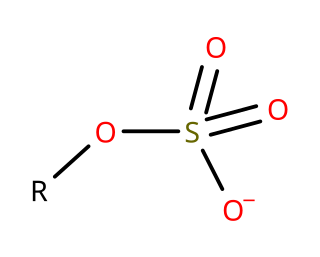
In organosulfur chemistry, organosulfates are a class of organic compounds sharing a common functional group with the structure R−O−SO−3. The SO4 core is a sulfate group and the R group is any organic residue. All organosulfates are formally esters derived from alcohols and sulfuric acid although many are not prepared in this way. Many sulfate esters are used in detergents, and some are useful reagents. Alkyl sulfates consist of a hydrophobic hydrocarbon chain, a polar sulfate group and either a cation or amine to neutralize the sulfate group. Examples include: sodium lauryl sulfate and related potassium and ammonium salts.
Oleochemistry is the study of vegetable oils and animal oils and fats, and oleochemicals derived from these fats and oils. The resulting product can be called oleochemicals (from Latin: oleum "olive oil"). The major product of this industry is soap, approximately 8.9×106 tons of which were produced in 1990. Other major oleochemicals include fatty acids, fatty acid methyl esters, fatty alcohols and fatty amines. Glycerol is a side product of all of these processes. Intermediate chemical substances produced from these basic oleochemical substances include alcohol ethoxylates, alcohol sulfates, alcohol ether sulfates, quaternary ammonium salts, monoacylglycerols (MAG), diacylglycerols (DAG), structured triacylglycerols (TAG), sugar esters, and other oleochemical products.
Omega-7 fatty acids are a class of unsaturated fatty acids in which the site of unsaturation is seven carbon atoms from the end of the carbon chain. The two most common omega-7 fatty acids in nature are palmitoleic acid and vaccenic acid. They are widely used in cosmetics due to their moisturizing properties. Omega-7 fats are not essential fatty acids in humans as they can be made endogenously. Diets rich in omega-7 fatty acids have been shown to have beneficial health effects, such as increasing levels of HDL cholesterol and lowering levels of LDL cholesterol.
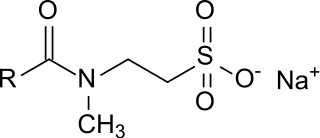
Taurates (or taurides) are a group of mild anionic surfactants. They are composed of a hydrophilic head group, consisting of N-methyltaurine (2-methylaminoethanesulfonic acid) and a lipophilic residue, consisting of a long-chain carboxylic acid (fatty acid), both linked via an amide bond. The fatty acids used could be lauric (C12), myristic (C14), palmitic (C16) or stearic acid (C18), but mainly mixtures of oleic acid (C18:1) and coconut fatty acid (C8 – C18) are used. Besides sodium, no other counterions play a relevant role (these could be e. g. ammonium or other alkali or alkaline earth metals).









