Related Research Articles

Waxes are a diverse class of organic compounds that are lipophilic, malleable solids near ambient temperatures. They include higher alkanes and lipids, typically with melting points above about 40 °C (104 °F), melting to give low viscosity liquids. Waxes are insoluble in water but soluble in nonpolar organic solvents such as hexane, benzene and chloroform. Natural waxes of different types are produced by plants and animals and occur in petroleum.

Surfactants are chemical compounds that decrease the surface tension or interfacial tension between two liquids, a liquid and a gas, or a liquid and a solid. The word "surfactant" is a blend of surface-active agent, coined in 1950. As they consist of a water-repellent and a water-attracting part, they enable water and oil to mix; they can form foam and facilitate the detachment of dirt.

Static electricity is an imbalance of electric charges within or on the surface of a material. The charge remains until it can move away by an electric current or electrical discharge. The word "static" is used to differentiate it from current electricity, where an electric charge flows through an electrical conductor.

In metallurgy, a flux is a chemical reducing agent, flowing agent, or purifying agent. Fluxes may have more than one function at a time. They are used in both extractive metallurgy and metal joining.

Conductive polymers or, more precisely, intrinsically conducting polymers (ICPs) are organic polymers that conduct electricity. Such compounds may have metallic conductivity or can be semiconductors. The main advantage of conductive polymers is that they are easy to process, mainly by dispersion. Conductive polymers are generally not thermoplastics, i.e., they are not thermoformable. But, like insulating polymers, they are organic materials. They can offer high electrical conductivity but do not show similar mechanical properties to other commercially available polymers. The electrical properties can be fine-tuned using the methods of organic synthesis and by advanced dispersion techniques.

Polyelectrolytes are polymers whose repeating units bear an electrolyte group. Polycations and polyanions are polyelectrolytes. These groups dissociate in aqueous solutions (water), making the polymers charged. Polyelectrolyte properties are thus similar to both electrolytes (salts) and polymers and are sometimes called polysalts. Like salts, their solutions are electrically conductive. Like polymers, their solutions are often viscous. Charged molecular chains, commonly present in soft matter systems, play a fundamental role in determining structure, stability and the interactions of various molecular assemblies. Theoretical approaches to describe their statistical properties differ profoundly from those of their electrically neutral counterparts, while technological and industrial fields exploit their unique properties. Many biological molecules are polyelectrolytes. For instance, polypeptides, glycosaminoglycans, and DNA are polyelectrolytes. Both natural and synthetic polyelectrolytes are used in a variety of industries.

Electrospinning is a fiber production method that uses electrical force to draw charged threads of polymer solutions for producing nanofibers with diameters ranging from nanometers to micrometers. Electrospinning shares characteristics of both electrospraying and conventional solution dry spinning of fibers. The process does not require the use of coagulation chemistry or high temperatures to produce solid threads from solution. This makes the process particularly suited to the production of fibers using large and complex molecules. Electrospinning from molten precursors is also practiced; this method ensures that no solvent can be carried over into the final product.

Electrophoretic deposition (EPD), is a term for a broad range of industrial processes which includes electrocoating, cathodic electrodeposition, anodic electrodeposition, and electrophoretic coating, or electrophoretic painting. A characteristic feature of this process is that colloidal particles suspended in a liquid medium migrate under the influence of an electric field (electrophoresis) and are deposited onto an electrode. All colloidal particles that can be used to form stable suspensions and that can carry a charge can be used in electrophoretic deposition. This includes materials such as polymers, pigments, dyes, ceramics and metals.
A fabric softener or fabric conditioner is a conditioner applied to laundry after it has been washed in a washing machine. A similar, more dilute preparation meant to be applied to dry fabric is known as a wrinkle releaser.
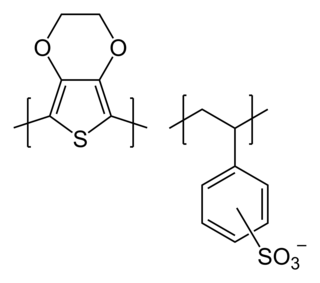
Poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS) is a composite material where PEDOT provides electrical conductivity, and PSS acts as a counter-ion to balance the charge and improve the water solubility and processability of PEDOT. Polystyrene sulfonate is a sulfonated polystyrene. Part of the sulfonyl groups are deprotonated and carry a negative charge. The other component poly(3,4-ethylenedioxythiophene) (PEDOT) is a conjugated polymer and carries positive charges and is based on polythiophene. Together the charged macromolecules form a macromolecular salt.

Hot-melt adhesive (HMA), also known as hot glue, is a form of thermoplastic adhesive that is commonly sold as solid cylindrical sticks of various diameters designed to be applied using a hot glue gun. The gun uses a continuous-duty heating element to melt the plastic glue, which the user pushes through the gun either with a mechanical trigger mechanism on the gun, or with direct finger pressure. The glue squeezed out of the heated nozzle is initially hot enough to burn and even blister skin. The glue is sticky when hot, and solidifies in a few seconds to one minute. Hot-melt adhesives can also be applied by dipping or spraying, and are popular with hobbyists and crafters both for affixing and as an inexpensive alternative to resin casting.
Ethylammonium nitrate or ethylamine nitrate (EAN) is a salt with formula [CH3CH2NH3]+[NO3]−. It is an odorless and colorless to slightly yellowish liquid with a melting point of 12 °C. This compound was described by Paul Walden in 1914, and is believed to be the earliest reported example of a room-temperature ionic liquid.

An antistatic device is any device that reduces, dampens, or otherwise inhibits electrostatic discharge, or ESD, which is the buildup or discharge of static electricity. ESD can damage electrical components such as computer hard drives, and even ignite flammable liquids and gases.
Dinonylnaphthylsulfonic acid (DINNSA) is an organic chemical, an aryl sulfonic acid. Its melting point is 259.5 °C and its boiling point is 600.4 °C. It has very low water solubility. It is a moderate skin irritant and a strong eye irritant. It has low volatility and vapor pressure and is stable above 100 °C.

An antistatic bag is a bag used for storing electronic components, which are prone to damage caused by electrostatic discharge (ESD).
Glycerol monostearate, commonly known as GMS, is a monoglyceride commonly used as an emulsifier in foods. It takes the form of a white, odorless, and sweet-tasting flaky powder that is hygroscopic. Chemically it is the glycerol ester of stearic acid. It is also used as hydration powder in exercise formulas
Paint has four major components: pigments, binders, solvents, and additives. Pigments serve to give paint its color, texture, toughness, as well as determining if a paint is opaque or not. Common white pigments include titanium dioxide and zinc oxide. Binders are the film forming component of a paint as it dries and affects the durability, gloss, and flexibility of the coating. Polyurethanes, polyesters, and acrylics are all examples of common binders. The solvent is the medium in which all other components of the paint are dissolved and evaporates away as the paint dries and cures. The solvent also modifies the curing rate and viscosity of the paint in its liquid state. There are two types of paint: solvent-borne and water-borne paints. Solvent-borne paints use organic solvents as the primary vehicle carrying the solid components in a paint formulation, whereas water-borne paints use water as the continuous medium. The additives that are incorporated into paints are a wide range of things which impart important effects on the properties of the paint and the final coating. Common paint additives are catalysts, thickeners, stabilizers, emulsifiers, texturizers, biocides to fight bacterial growth, etc.
Adsorption of polyelectrolytes on solid substrates is a surface phenomenon where long-chained polymer molecules with charged groups bind to a surface that is charged in the opposite polarity. On the molecular level, the polymers do not actually bond to the surface, but tend to "stick" to the surface via intermolecular forces and the charges created by the dissociation of various side groups of the polymer. Because the polymer molecules are so long, they have a large amount of surface area with which to contact the surface and thus do not desorb as small molecules are likely to do. This means that adsorbed layers of polyelectrolytes form a very durable coating. Due to this important characteristic of polyelectrolyte layers they are used extensively in industry as flocculants, for solubilization, as supersorbers, antistatic agents, as oil recovery aids, as gelling aids in nutrition, additives in concrete, or for blood compatibility enhancement to name a few.

Electrostatic discharge materials are plastics that reduce static electricity to protect against damage to electrostatic-sensitive devices (ESD) or to prevent the accidental ignition of flammable liquids or gases.

OCSiAl is a global nanotechnology company, the world's largest graphene nanotube manufacturer, conducting its operations worldwide. The OCSiAl headquarters are located in Luxembourg, with several offices in the United States, Europe and Asia.
References
- ↑ Robinson, K; Durkin, W (2010). "Electrostatic issues in roll-to-roll manufacturing operations". IEEE Transactions on Industry Applications. 46 (6): 2172–2178. doi:10.1109/TIA.2010.2071270. S2CID 14320690.
- ↑ Shelton, S E (April 2004). "In-mold labeling: electrostatics are the way to go". Plastics Technology.
- ↑ J Markarian, New developments in antistatic and conductive additives, Plastics Additives and Compounding, September / October 2008, 22-25.
- ↑ Gornicka, B (2010). "Antistatic Properties of Nanofilled Coatings". Acta Physica Polonica A. 117 (5): 869–872. Bibcode:2010AcPPA.117..869G. doi: 10.12693/APhysPolA.117.869 .
- ↑ Patent DE 102006045869, Antistatic treatment for coatings, e.g. paints, printing inks and lacquers, comprises using an ionic liquid or a solution of a salt in an ionic liquid as the antistatic agent, April 3, 2008.
- ↑ Marquardt, Kurt (2011). "Textile Auxiliaries, 2. Auxiliaries for Fiber Production and Processing". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.o26_o06. ISBN 978-3-527-30385-4.
- ↑ "Stadis (R) 450 Material Safety Data Sheet" (PDF). apsaviation.com.
- ↑ "Stadis 425(id:8508862) Product details - View Stadis 425 from Hans Group LTD(Shanghai Representative Office) - EC21".
- ↑ Lambourne, R.; Strivens, T. A. (1999-08-23). Paint and Surface Coatings: Theory and Practice. Elsevier. ISBN 9781855737006.
- ↑ "Enhanced antistatic additives for hydrocarbon fuels & solvents".