
Phalaris arundinacea, or reed canary grass, is a tall, perennial bunchgrass that commonly forms extensive single-species stands along the margins of lakes and streams and in wet open areas, with a wide distribution in Europe, Asia, northern Africa and North America. Other common names for the plant include gardener's-garters and ribbon grass in English, alpiste roseau in French, Rohrglanzgras in German, kusa-yoshi in Japanese, caniço-malhado in Portuguese, and hierba cinta and pasto cinto in Spanish.

Ruta graveolens, commonly known as rue, common rue or herb-of-grace, is a species of the genus Ruta grown as an ornamental plant and herb. It is native to the Balkan Peninsula. It is grown throughout the world in gardens, especially for its bluish leaves, and sometimes for its tolerance of hot and dry soil conditions. It is also cultivated as a culinary herb, and to a lesser extent as an insect repellent and incense.
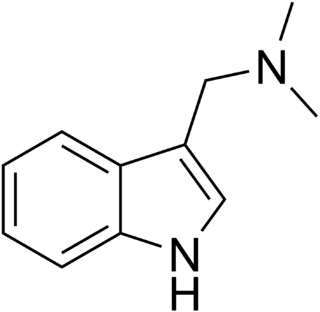
Gramine is a naturally occurring indole alkaloid present in several plant species. Gramine may play a defensive role in these plants, since it is toxic to many organisms.

Hordenine is an alkaloid of the phenethylamine class that occurs naturally in a variety of plants, taking its name from one of the most common, barley. Chemically, hordenine is the N-methyl derivative of N-methyltyramine, and the N,N-dimethyl derivative of the well-known biogenic amine tyramine, from which it is biosynthetically derived and with which it shares some pharmacological properties. As of September 2012, hordenine is widely sold as an ingredient of nutritional supplements, with the claims that it is a stimulant of the central nervous system, and has the ability to promote weight loss by enhancing metabolism. In experimental animals, given sufficiently large doses parenterally, hordenine does produce an increase in blood pressure, as well as other disturbances of the cardiovascular, respiratory, and nervous systems. These effects are generally not reproduced by oral administration of the drug in test animals, and virtually no scientific reports of the effects of hordenine in human beings have been published.

Mitraphylline, an oxindole derivative, is an active alkaloid in the leaves of the tree Mitragyna speciosa, commonly known as kratom. As a non-narcotic constituent, it also occurs to a significant amount in the bark of Uncaria tomentosa along with a number of isomeric alkaloids.

Oxindole (2-indolone) is an aromatic heterocyclic organic compound with the formula C6H4CHC(O)NH. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing ring. Oxindole is a modified indoline with a substituted carbonyl at the second position of the 5-member indoline ring. Classified as a cyclic amide, it is a pale yellow solid.

A cardenolide is a type of steroid. Many plants contain derivatives, collectively known as cardenolides, including many in the form of cardenolide glycosides (cardenolides that contain structural groups derived from sugars). Cardenolide glycosides are often toxic; specifically, they are heart-arresting. Cardenolides are toxic to animals through inhibition of the enzyme Na+/K+‐ATPase, which is responsible for maintaining the sodium and potassium ion gradients across the cell membranes.

Senegalia berlandieri is a shrub native to the Southwestern United States and northeast Mexico that belongs to the Mimosoid clade of Fabaceae. It grows 1 to 5 metres tall, with blossoms that are spherical and white, occurring from February through April. The berlandieri epithet comes from the name of Jean-Louis Berlandier, a French naturalist who studied wildlife native to Texas and Mexico. S. berlandieri contains a wide variety of alkaloids and has been known to cause toxic reactions in domestic animals such as goats.

Alstonia scholaris, commonly called blackboard tree, scholar tree, milkwood or devil's tree in English, is an evergreen tropical tree in the Dogbane Family (Apocynaceae). It is native to southern China, tropical Asia and Australasia, where it is a common ornamental plant. It is a toxic plant, but is used traditionally for myriad diseases and complaints.
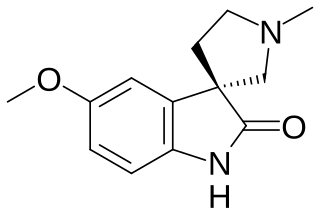
Horsfiline is an oxindole alkaloid found in the plant Horsfieldia superba, which is used in traditional herbal medicine. It has analgesic effects and has been the subject of research both to produce it synthetically by convenient routes and to develop analogues and derivatives which may have improved analgesic effects.

Tetrandrine, a bis-benzylisoquinoline alkaloid, is a calcium channel blocker. It is isolated from the plant Stephania tetrandra, and other Chinese and Japanese herbs.

Indole is an organic compound with the formula C6H4CCNH3. Indole is classified as an aromatic heterocycle. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indoles are derivatives of indole where one or more of the hydrogen atoms have been replaced by substituent groups. Indoles are widely distributed in nature, most notably as amino acid tryptophan and neurotransmitter serotonin.

Senecionine is a toxic pyrrolizidine alkaloid isolated from various botanical sources. It takes its name from the Senecio genus and is produced by many different plants in that genus, including Jacobaea vulgaris. It has also been isolated from several other plants, including Brachyglottis repanda, Emilia, Erechtites hieraciifolius, Petasites, Syneilesis, Crotalaria, Caltha leptosepala, and Castilleja.
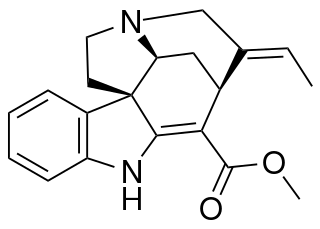
Akuammicine is a monoterpene indole alkaloid of the Vinca sub-group. It is found in the Apocynaceae family of plants including Picralima nitida, Vinca minor and the Aspidosperma.

Quassinoids are degraded triterpene lactones of the Simaroubaceae plant family grouped into C-18, C-19, C-20, C-22 and C-25 types. The prototypical member of the group, quassin, was first described in the 19th century from plants of the genus Quassia from which it gets its name. It was isolated in 1937, and its structure elucidated in 1961.
Penicillium paxilli is an anamorph, saprophytic species of the genus Penicillium which produces paxilline, paxisterol, penicillone, pyrenocine A, paspaline B and verruculogene. Penicillium paxilli is used as a model to study the biochemistry of the indol-diterepene biosynthesis

Tabernaemontanine is a naturally occurring monoterpene indole alkaloid found in several species in the genus Tabernaemontana including Tabernaemontana divaricata.

Speciociliatine is a major alkaloid of the plant Mitragyna speciosa, commonly known as kratom. It is a stereoisomer of Mitragynine and constitutes 0.00156 - 2.9% of the dried leaf material.
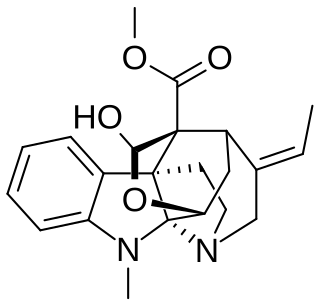
Corymine, also known as NSC381080, is a natural alkaloid found in Hunteria zeylanica.


















