The 1,3-dipolar cycloaddition is a chemical reaction between a 1,3-dipole and a dipolarophile to form a five-membered ring. The earliest 1,3-dipolar cycloadditions were described in the late 19th century to the early 20th century, following the discovery of 1,3-dipoles. Mechanistic investigation and synthetic application were established in the 1960s, primarily through the work of Rolf Huisgen. Hence, the reaction is sometimes referred to as the Huisgen cycloaddition. 1,3-dipolar cycloaddition is an important route to the regio- and stereoselective synthesis of five-membered heterocycles and their ring-opened acyclic derivatives. The dipolarophile is typically an alkene or alkyne, but can be other pi systems. When the dipolarophile is an alkyne, aromatic rings are generally produced.

The Martinet dioxindole synthesis was first reported in 1913 by J. Martinet. It is a chemical reaction in which a primary or secondary aniline or substituted aromatic amine is condensed with ethyl or methyl ester of mesoxalic acid to make a dioxindole in the absence of oxygen.

Azomethine ylides are nitrogen-based 1,3-dipoles, consisting of an iminium ion next to a carbanion. They are used in 1,3-dipolar cycloaddition reactions to form five-membered heterocycles, including pyrrolidines and pyrrolines. These reactions are highly stereo- and regioselective, and have the potential to form four new contiguous stereocenters. Azomethine ylides thus have high utility in total synthesis, and formation of chiral ligands and pharmaceuticals. Azomethine ylides can be generated from many sources, including aziridines, imines, and iminiums. They are often generated in situ, and immediately reacted with dipolarophiles.

The Prato reaction is a particular example of the well-known 1,3-dipolar cycloaddition of azomethine ylides to olefins. In fullerene chemistry this reaction refers to the functionalization of fullerenes and nanotubes. The amino acid sarcosine reacts with paraformaldehyde when heated at reflux in toluene to an ylide which reacts with a double bond in a 6,6 ring position in a fullerene via a 1,3-dipolar cycloaddition to yield a N-methylpyrrolidine derivative or pyrrolidinofullerene or pyrrolidino[[3,4:1,2]] [60]fullerene in 82% yield based on C60 conversion.

In organic chemistry, aziridines are organic compounds containing the aziridine functional group, a three-membered heterocycle with one amine and two methylene bridges. The parent compound is aziridine, with molecular formula C2H4NH. Several drugs feature aziridine rings, including mitomycin C, porfiromycin, and azinomycin B (carzinophilin).
The Negishi coupling is a widely employed transition metal catalyzed cross-coupling reaction. The reaction couples organic halides or triflates with organozinc compounds, forming carbon-carbon bonds (C-C) in the process. A palladium (0) species is generally utilized as the metal catalyst, though nickel is sometimes used. A variety of nickel catalysts in either Ni0 or NiII oxidation state can be employed in Negishi cross couplings such as Ni(PPh3)4, Ni(acac)2, Ni(COD)2 etc.
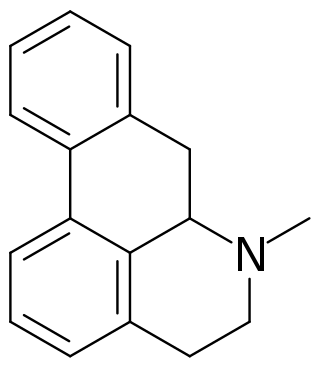
Aporphine is an alkaloid with the chemical formula C17H17N. It is the core chemical substructure of the aporphine alkaloids, a subclass of quinoline alkaloids. It can exist in either of two enantiomeric forms, (R)-aporphine and (S)-aporphine.

In organic chemistry, organocatalysis is a form of catalysis in which the rate of a chemical reaction is increased by an organic catalyst. This "organocatalyst" consists of carbon, hydrogen, sulfur and other nonmetal elements found in organic compounds. Because of their similarity in composition and description, they are often mistaken as a misnomer for enzymes due to their comparable effects on reaction rates and forms of catalysis involved.
Isoindoline is a heterocyclic organic compound with the molecular formula C8H9N. The parent compound has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing ring. The compound's structure is similar to indoline except that the nitrogen atom is in the 2 position instead of the 1 position of the five-membered ring. Isoindoline itself is not commonly encountered, but several derivatives are found in nature and some synthetic derivatives are commercially valuable drugs, e.g. pazinaclone.

Spirotryprostatin B is an indolic alkaloid found in the Aspergillus fumigatus fungus that belongs to a class of naturally occurring 2,5-diketopiperazines. Spirotryprostatin B and several other indolic alkaloids have been found to have anti-mitotic properties, and as such they have become of great interest as anti-cancer drugs. Because of this, the total syntheses of these compounds is a major pursuit of organic chemists, and a number of different syntheses have been published in the chemical literature.

Horsfieldia is a genus of evergreen trees. The genus consists of about 100 species and is distributed across South Asia, from India to the Philippines and Papua New Guinea. Some species are used for timber. Species in the genus sometimes contain alkaloids, including horsfiline, which has analgesic effects.

Horsfieldia superba is a species of plant in the family Myristicaceae. It is a tree found in Sumatra, Peninsular Malaysia, and Singapore, and is threatened by habitat loss. It is used in traditional herbal medicine and contains an alkaloid called horsfiline, which has analgesic effects, as well as several other compounds including 5-MeO-DMT and 6-methoxy-2-methyl-1,2,3,4-tetrahydro-β-carboline.
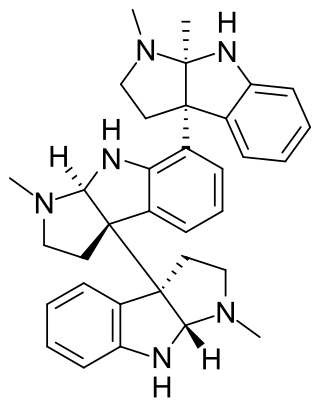
Hodgkinsine is an alkaloid found in plants of the genus Psychotria, particularly Psychotria colorata, although it is also found in Psychotria lyciiflora and probably other species in this family,

Indole is an organic compound with the formula C6H4CCNH3. Indole is classified as an aromatic heterocycle. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indoles are derivatives of indole where one or more of the hydrogen atoms have been replaced by substituent groups. Indoles are widely distributed in nature, most notably as amino acid tryptophan and neurotransmitter serotonin.

Oxazoline is a five-membered heterocyclic organic compound with the formula C3H5NO. It is the parent of a family of compounds called oxazolines, which contain non-hydrogenic substituents on carbon and/or nitrogen. Oxazolines are the unsaturated analogues of oxazolidines, and they are isomeric with isoxazolines, where the N and O are directly bonded. Two isomers of oxazoline are known, depending on the location of the double bond.
Within the area of organocatalysis, (thio)urea organocatalysis describes the use of ureas and thioureas to accelerate and stereochemically alter organic transformations. The effects arise through hydrogen-bonding interactions between the substrate and the (thio)urea. Unlike classical catalysts, these organocatalysts interact by non-covalent interactions, especially hydrogen bonding. The scope of these small-molecule H-bond donors termed (thio)urea organocatalysis covers both non-stereoselective and stereoselective reactions.

Conolidine is an indole alkaloid. Preliminary reports suggest that it could provide analgesic effects with few of the detrimental side-effects associated with opioids such as morphine, though at present it has only been evaluated in mouse models.

Synthesis of morphine-like alkaloids in chemistry describes the total synthesis of the natural morphinan class of alkaloids that includes codeine, morphine, oripavine, and thebaine and the closely related semisynthetic analogs methorphan, buprenorphine, hydromorphone, hydrocodone, isocodeine, naltrexone, nalbuphine, oxymorphone, oxycodone, and naloxone.
Modified Wittig–Claisen tandem reaction is a cascade reaction that combines the Wittig reaction and Claisen rearrangement together. The Wittig reaction generates the allyl vinyl ether intermediate that further participates in a Claisen rearrangement to generate the final γ,δ-unsaturated ketone or aldehyde product.

Jieping Zhu is an organic chemist specializing in natural product total synthesis and organometallics. He is a professor of chemistry at EPFL and the head of the Laboratory of Synthesis and Natural Products.















