Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans.
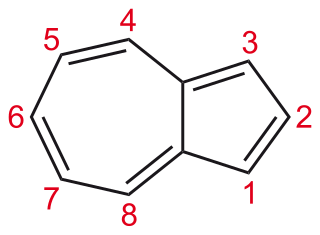
Azulene is an organic compound and an isomer of naphthalene. Naphthalene is colourless, whereas azulene is dark blue. The compound is named after this colour, as "azul" is Spanish for blue. Two terpenoids, vetivazulene (4,8-dimethyl-2-isopropylazulene) and guaiazulene (1,4-dimethyl-7-isopropylazulene), that feature the azulene skeleton are found in nature as constituents of pigments in mushrooms, guaiac wood oil, and some marine invertebrates.

Pyridinium chlorochromate (PCC) is a yellow-orange salt with the formula [C5H5NH]+[CrO3Cl]−. It is a reagent in organic synthesis used primarily for oxidation of alcohols to form carbonyls. A variety of related compounds are known with similar reactivity. PCC offers the advantage of the selective oxidation of alcohols to aldehydes or ketones, whereas many other reagents are less selective.
Grubbs catalysts are a series of transition metal carbene complexes used as catalysts for olefin metathesis. They are named after Robert H. Grubbs, the chemist who supervised their synthesis. Several generations of the catalyst have also been developed. Grubbs catalysts tolerate many functional groups in the alkene substrates, are air-tolerant, and are compatible with a wide range of solvents. For these reasons, Grubbs catalysts have become popular in synthetic organic chemistry. Grubbs, together with Richard R. Schrock and Yves Chauvin, won the Nobel Prize in Chemistry in recognition of their contributions to the development of olefin metathesis.

Lawesson's reagent (LR) is a chemical compound used in organic synthesis as a thiation agent. Lawesson's reagent was first made popular by Sven-Olov Lawesson, who did not, however, invent it. Lawesson's reagent was first made in 1956 during a systematic study of the reactions of arenes with P4S10.
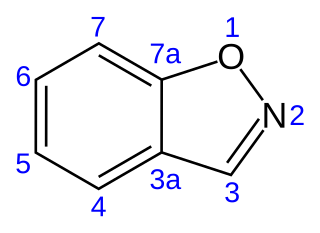
1,2-Benzisoxazole is an aromatic organic compound with a molecular formula C7H5NO containing a benzene-fused isoxazole ring structure. The compound itself has no common applications; however, functionalized benzisoxazoles and benzisoxazoyls have a variety of uses, including pharmaceutical drugs such as some antipsychotics (including risperidone, paliperidone, ocaperidone, and iloperidone) and the anticonvulsant zonisamide.
The Niementowski quinazoline synthesis is the chemical reaction of anthranilic acids with amides to form 4-oxo-3,4-dihydroquinazolines (3H-quinazolin-4-ones).
The Étard reaction is a chemical reaction that involves the direct oxidation of an aromatic or heterocyclic bound methyl group to an aldehyde using chromyl chloride. For example, toluene can be oxidized to benzaldehyde.
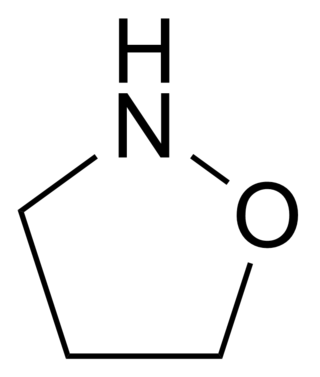
Isoxazolidine is the organic compound with the formula (CH2)3(NH)O. It is the parent of a family of compounds called Isoxazolidines, which are saturated C3NO heterocyclic rings where the nitrogen and oxygen occupy adjacent positions (1 and 2). They are the saturated analogues of Isoxazoles, and they are isomeric with oxazolidines, where the N and O are separated by one carbon.

Organobismuth chemistry is the chemistry of organometallic compounds containing a carbon to bismuth chemical bond. Applications are few. The main bismuth oxidation states are Bi(III) and Bi(V) as in all higher group 15 elements. The energy of a bond to carbon in this group decreases in the order P > As > Sb > Bi. The first reported use of bismuth in organic chemistry was in oxidation of alcohols by Frederick Challenger in 1934 (using Ph3Bi(OH)2). Knowledge about methylated species of bismuth in environmental and biological media is limited.

DuPhos is a class of organophosphorus compound that are used ligands for asymmetric synthesis. The name DuPhos is derived from (1) the chemical company that sponsored the research leading to this ligand's invention, DuPont and (2) the compound is a diphosphine ligand type. Specifically it is classified as a C2-symmetric ligand, consisting of two phospholanes rings affixed to a benzene ring.
The imine Diels–Alder reaction involves the transformation of all-carbon dienes and imine dienophiles into tetrahydropyridines.
The Minisci reaction is a named reaction in organic chemistry. It is a nucleophilic radical substitution to an electron deficient aromatic compound, most commonly the introduction of an alkyl group to a nitrogen containing heterocycle. The reaction was published in 1971 by F. Minisci. In the case of N-Heterocycles, the conditions must be acidic to ensure protonation of said heterocycle. A typical reaction is that between pyridine and pivalic acid with silver nitrate, sulfuric acid and ammonium persulfate to form 2-tert-butylpyridine. The reaction resembles Friedel-Crafts alkylation but with opposite reactivity and selectivity.

Phosphinooxazolines are a class of chiral ligands used in asymmetric catalysis. Their complexes are particularly effective at generating single enatiomers in reactions involving highly symmetric transition states, such as allylic substitutions, which are typically difficult to perform stereoselectively. The ligands are bidentate and have been shown to be hemilabile with the softer P‑donor being more firmly bound than the harder N‑donor.

5-Hydroxy-2(5H)-furanone is a furanone derived from oxidation of furfural using singlet oxygen. This oxidation is carried out generally in methanol or ethanol with a sensitizer like methylene blue or Rose bengal. The mechanism of this reaction is depicted as below.
Mesomeric betaines are dipolar heterocyclic compounds in which both the negative and the positive charges are delocalized.

Diethyl oxomalonate is the diethyl ester of mesoxalic acid (ketomalonic acid), the simplest oxodicarboxylic acid and thus the first member (n = 0) of a homologous series HOOC–CO–(CH2)n–COOH with the higher homologues oxalacetic acid (n = 1), α-ketoglutaric acid (n = 2) and α-ketoadipic acid (n = 3) (the latter a metabolite of the amino acid lysine). Diethyl oxomalonate reacts because of its highly polarized keto group as electrophile in addition reactions and is a highly active reactant in pericyclic reactions such as the Diels-Alder reactions, cycloadditions or ene reactions. At humid air, mesoxalic acid diethyl ester reacts with water to give diethyl mesoxalate hydrate and the green-yellow oil are spontaneously converted to white crystals.
In organic chemistry, the Davis oxidation or Davis' oxaziridine oxidation refers to oxidations involving the use of the Davis reagent or other similar oxaziridine reagents. This reaction mainly refers to the generation of α-hydroxy carbonyl compounds (acyloins) from ketones or esters. The reaction is carried out in a basic environment to generate the corresponding enolate from the ketone or ester. This reaction has been shown to work for amides.
In organic chemistry, the Lombardo methylenation is a name reaction that allows for the methylenation of carbonyl compounds with the use of Lombardo's reagent, which is a mix of zinc, dibromomethane, and titanium tetrachloride.

Hafnium(IV) triflate or hafnium trifluoromethansulfonate is an inorganic substance with the idealized formula Hf(OSO2CF3)4, also written as Hf(OTf)4. Hafnium triflate is used as an impure mixture as a catalyst. Hafnium (IV) has an ionic radius of intermediate range (Al < Ti < Hf < Zr < Sc < Ln) and has an oxophilic hard character typical of group IV metals. This solid is a stronger Lewis acid than its typical precursor hafnium tetrachloride, HfCl4, because of the strong electron-withdrawing nature of the four triflate groups, which makes it a great Lewis acid and has many uses including as a great catalyst at low Lewis acid loadings for electrophilic aromatic substitution and nucleophilic substitution reactions.











