Atomic physics is the field of physics that studies atoms as an isolated system of electrons and an atomic nucleus. It is primarily concerned with the arrangement of electrons around the nucleus and the processes by which these arrangements change. This comprises ions, neutral atoms and, unless otherwise stated, it can be assumed that the term atom includes ions.

Spectroscopy is the study of the interaction between matter and electromagnetic radiation. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, by a prism. Later the concept was expanded greatly to include any interaction with radiative energy as a function of its wavelength or frequency, predominantly in the electromagnetic spectrum, though matter waves and acoustic waves can also be considered forms of radiative energy; recently, with tremendous difficulty, even gravitational waves have been associated with a spectral signature in the context of LIGO and laser interferometry. Spectroscopic data are often represented by an emission spectrum, a plot of the response of interest as a function of wavelength or frequency.

A quantum mechanical system or particle that is bound—that is, confined spatially—can only take on certain discrete values of energy. This contrasts with classical particles, which can have any energy. These discrete values are called energy levels. The term is commonly used for the energy levels of electrons in atoms, ions, or molecules, which are bound by the electric field of the nucleus, but can also refer to energy levels of nuclei or vibrational or rotational energy levels in molecules. The energy spectrum of a system with such discrete energy levels is said to be quantized.

In astronomy, the interstellar medium (ISM) is the matter and radiation that exists in the space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, as well as dust and cosmic rays. It fills interstellar space and blends smoothly into the surrounding intergalactic space. The energy that occupies the same volume, in the form of electromagnetic radiation, is the interstellar radiation field.

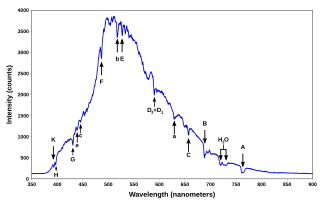
The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an atom or molecule making a transition from a high energy state to a lower energy state. The photon energy of the emitted photon is equal to the energy difference between the two states. There are many possible electron transitions for each atom, and each transition has a specific energy difference. This collection of different transitions, leading to different radiated wavelengths, make up an emission spectrum. Each element's emission spectrum is unique. Therefore, spectroscopy can be used to identify the elements in matter of unknown composition. Similarly, the emission spectra of molecules can be used in chemical analysis of substances.

The Franck–Hertz experiment was the first electrical measurement to clearly show the quantum nature of atoms, and thus "transformed our understanding of the world". It was presented on April 24, 1914, to the German Physical Society in a paper by James Franck and Gustav Hertz. Franck and Hertz had designed a vacuum tube for studying energetic electrons that flew through a thin vapor of mercury atoms. They discovered that, when an electron collided with a mercury atom, it could lose only a specific quantity of its kinetic energy before flying away. This energy loss corresponds to decelerating the electron from a speed of about 1.3 million meters per second to zero. A faster electron does not decelerate completely after a collision, but loses precisely the same amount of its kinetic energy. Slower electrons merely bounce off mercury atoms without losing any significant speed or kinetic energy.
Plasma diagnostics are a pool of methods, instruments, and experimental techniques used to measure properties of a plasma, such as plasma components' density, distribution function over energy (temperature), their spatial profiles and dynamics, which enable to derive plasma parameters.

In quantum mechanics, an excited state of a system is any quantum state of the system that has a higher energy than the ground state. Excitation is an elevation in energy level above an arbitrary baseline energy state. In physics there is a specific technical definition for energy level which is often associated with an atom being raised to an excited state. The temperature of a group of particles is indicative of the level of excitation.

Internal conversion is a radioactive decay process wherein an excited nucleus interacts electromagnetically with one of the orbital electrons of the atom. This causes the electron to be emitted (ejected) from the atom. Thus, in an internal conversion process, a high-energy electron is emitted from the radioactive atom, but not from the nucleus. For this reason, the high-speed electrons resulting from internal conversion are not called beta particles, since the latter come from beta decay, where they are newly created in the nuclear decay process.

A glow discharge is a plasma formed by the passage of electric current through a gas. It is often created by applying a voltage between two electrodes in a glass tube containing a low-pressure gas. When the voltage exceeds a value called the striking voltage, the gas ionization becomes self-sustaining, and the tube glows with a colored light. The color depends on the gas used.
In chemistry, nuclear physics, and particle physics, inelastic scattering is a fundamental scattering process in which the kinetic energy of an incident particle is not conserved. In an inelastic scattering process, some of the energy of the incident particle is lost or increased. Although the term is historically related to the concept of inelastic collision in dynamics, the two concepts are quite distinct; inelastic collision in dynamics refers to processes in which the total macroscopic kinetic energy is not conserved. In general, scattering due to inelastic collisions will be inelastic, but, since elastic collisions often transfer kinetic energy between particles, scattering due to elastic collisions can also be inelastic, as in Compton scattering.
In spectroscopy, a forbidden mechanism is a spectral line associated with absorption or emission of light by atomic nuclei, atoms, or molecules which undergo a transition that is not allowed by a particular selection rule but is allowed if the approximation associated with that rule is not made. For example, in a situation where, according to usual approximations, the process cannot happen, but at a higher level of approximation the process is allowed but at a much lower rate.
Ultrafast laser spectroscopy is a spectroscopic technique that uses ultrashort pulse lasers for the study of dynamics on extremely short time scales. Different methods are used to examine dynamics of charge carriers, atoms and molecules. Many different procedures have been developed spanning different time scales and photon energy ranges; some common methods are listed below.

Doppler cooling is a mechanism that can be used to trap and slow the motion of atoms to cool a substance. The term is sometimes used synonymously with laser cooling, though laser cooling includes other techniques.
Photoelectrochemical processes are processes in photoelectrochemistry; they usually involve transforming light into other forms of energy. These processes apply to photochemistry, optically pumped lasers, sensitized solar cells, luminescence, and photochromism.
In astronomy and in astrophysics, for radiative losses of the solar corona, it is meant the energy flux radiated from the external atmosphere of the Sun, and, in particular, the processes of production of the radiation coming from the solar corona and transition region, where the plasma is optically-thin. On the contrary, in the chromosphere, where the temperature decreases from the photospheric value of 6000 K to the minimum of 4400 K, the optical depth is about 1, and the radiation is thermal.

Collision-induced dissociation (CID), also known as collisionally activated dissociation (CAD), is a mass spectrometry technique to induce fragmentation of selected ions in the gas phase. The selected ions are usually accelerated by applying an electrical potential to increase the ion kinetic energy and then allowed to collide with neutral molecules. In the collision some of the kinetic energy is converted into internal energy which results in bond breakage and the fragmentation of the molecular ion into smaller fragments. These fragment ions can then be analyzed by tandem mass spectrometry.

A streamer discharge, also known as filamentary discharge, is a type of transient electrical discharge. Streamer discharges can form when an insulating medium is exposed to a large potential difference. When the electric field created by the applied voltage is sufficiently large, accelerated electrons strike air molecules with enough energy to knock other electrons off them, ionizing them, and the freed electrons go on to strike more molecules in a chain reaction. These electron avalanches create ionized, electrically conductive regions in the air near the electrode creating the electric field. The space charge created by the electron avalanches gives rise to an additional electric field. This field can enhance the growth of new avalanches in a particular direction. Then the ionized region grows quickly in that direction, forming a finger-like discharge called a streamer.
An excimer lamp is a source of ultraviolet light produced by spontaneous emission of excimer (exciplex) molecules.