Lysergic acid, also known as D-lysergic acid and (+)-lysergic acid, is a precursor for a wide range of ergoline alkaloids that are produced by the ergot fungus and found in the seeds of Turbina corymbosa (ololiuhqui), Argyreia nervosa, and Ipomoea tricolor.
The Pictet–Spengler reaction is a chemical reaction in which a β-arylethylamine undergoes condensation with an aldehyde or ketone followed by ring closure. The reaction was first discovered in 1911 by Amé Pictet and Theodor Spengler. Traditionally, an acidic catalyst in protic solvent was employed with heating; however, the reaction has been shown to work in aprotic media in superior yields and sometimes without acid catalysis. The Pictet–Spengler reaction can be considered a special case of the Mannich reaction, which follows a similar reaction pathway. The driving force for this reaction is the electrophilicity of the iminium ion generated from the condensation of the aldehyde and amine under acid conditions. This explains the need for an acid catalyst in most cases, as the imine is not electrophilic enough for ring closure but the iminium ion is capable of undergoing the reaction.

Indole-3-acetic acid is the most common naturally occurring plant hormone of the auxin class. It is the best known of the auxins, and has been the subject of extensive studies by plant physiologists. IAA is a derivative of indole, containing a carboxymethyl substituent. It is a colorless solid that is soluble in polar organic solvents.
Cyclopiazonic acid (α-CPA), a mycotoxin and a fungal neurotoxin, is made by the molds Aspergillus and Penicillium. It is an indole-tetramic acid that serves as a toxin due to its ability to inhibit calcium-dependent ATPases found in the endoplasmic and sarcoplasmic reticulum. This inhibition disrupts the muscle contraction-relaxation cycle and the calcium gradient that is maintained for proper cellular activity in cells.

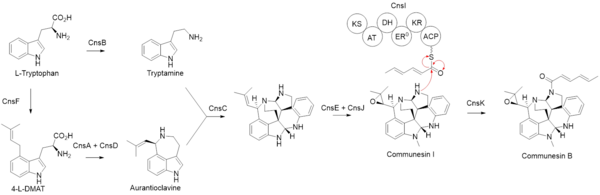
Indole alkaloids are a class of alkaloids containing a structural moiety of indole; many indole alkaloids also include isoprene groups and are thus called terpene indole or secologanin tryptamine alkaloids. Containing more than 4100 known different compounds, it is one of the largest classes of alkaloids. Many of them possess significant physiological activity and some of them are used in medicine. The amino acid tryptophan is the biochemical precursor of indole alkaloids.

Camptothecin (CPT) is a topoisomerase inhibitor. It was discovered in 1966 by M. E. Wall and M. C. Wani in systematic screening of natural products for anticancer drugs. It was isolated from the bark and stem of Camptotheca acuminata, a tree native to China used in traditional Chinese medicine. It has been used clinically more recently in China for the treatment of gastrointestinal tumors. CPT showed anticancer activity in preliminary clinical trials, especially against breast, ovarian, colon, lung, and stomach cancers. However, it has low solubility and adverse effects have been reported when used therapeutically, so synthetic and medicinal chemists have developed numerous syntheses of camptothecin and various derivatives to increase the benefits of the chemical, with good results. Four CPT analogues have been approved and are used in cancer chemotherapy today: topotecan, irinotecan, belotecan, and trastuzumab deruxtecan. Camptothecin has also been found in other plants including Chonemorpha fragrans.
Strictosidine synthase (EC 4.3.3.2) is an enzyme in alkaloid biosynthesis that catalyses the condensation of tryptamine with secologanin to form strictosidine in a formal Pictet–Spengler reaction:
Paxilline is a toxic, tremorgenic diterpene indole polycyclic alkaloid molecule produced by Penicillium paxilli which was first characterized in 1975. Paxilline is one of a class of tremorigenic mycotoxins, is a potassium channel blocker, and is potentially genotoxic.

Indolocarbazoles (ICZs) are a class of compounds that are under current study due to their potential as anti-cancer drugs and the prospective number of derivatives and uses found from the basic backbone alone. First isolated in 1977, a wide range of structures and derivatives have been found or developed throughout the world. Due to the extensive number of structures available, this review will focus on the more important groups here while covering their occurrence, biological activity, biosynthesis, and laboratory synthesis.

Ergocryptine is an ergopeptine and one of the ergoline alkaloids. It is isolated from ergot or fermentation broth and it serves as starting material for the production of bromocriptine. Two isomers of ergocryptine exist, α-ergocryptine and β-ergocryptine. The beta differs from the alpha form only in the position of a single methyl group, which is a consequence of the biosynthesis in which the proteinogenic amino acid leucine is replaced by isoleucine. β-Ergocryptine was first identified in 1967 by Albert Hofmann. Ergot from different sources have different ratios of the two isomers.

Indole is an aromatic, heterocyclic, organic compound with the formula C8H7N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore formation, plasmid stability, resistance to drugs, biofilm formation, and virulence. The amino acid tryptophan is an indole derivative and the precursor of the neurotransmitter serotonin.

Ajmalicine, also known as δ-yohimbine or raubasine, is an antihypertensive drug used in the treatment of high blood pressure. It has been marketed under numerous brand names including Card-Lamuran, Circolene, Cristanyl, Duxil, Duxor, Hydroxysarpon, Iskedyl, Isosarpan, Isquebral, Lamuran, Melanex, Raunatin, Saltucin Co, Salvalion, and Sarpan. It is an alkaloid found naturally in various plants such as Rauvolfia spp., Catharanthus roseus, and Mitragyna speciosa.

Lyngbyatoxin-a is a cyanotoxin produced by certain cyanobacteria species, most notably Moorea producens. It is produced as defense mechanism to ward off any would-be predators of the bacterium, being a potent blister agent as well as carcinogen. Low concentrations cause a common skin condition known as seaweed dermatitis.

Fumitremorgins are tremorogenic metabolites of Aspergillus and Penicillium, that belong to a class of naturally occurring 2,5-diketopiperazines.
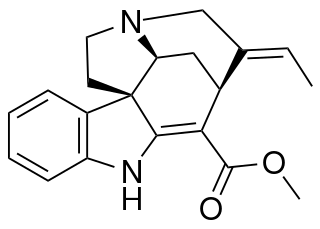
Akuammicine is a monoterpene indole alkaloid of the Vinca sub-group. It is found in the Apocynaceae family of plants including Picralima nitida, Vinca minor and the Aspidosperma.
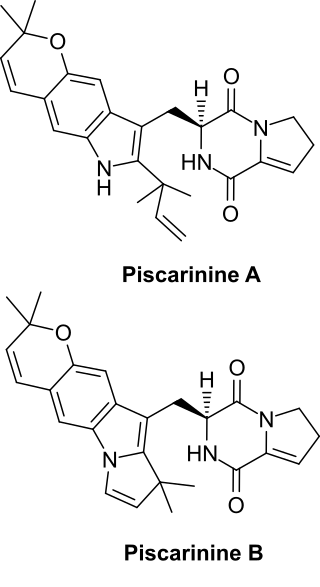
Piscarinines are bioactive alkaloid isolates of Penicillium piscarium NKM F-961 and Penicillium piscarium Westling that belong to a class of naturally occurring 2,5-diketopiperazines. The cytotoxic dehydroproline tryptophan derivatives piscarinines A and B were shown to be active against the prostate cancer cell line LNCAP.
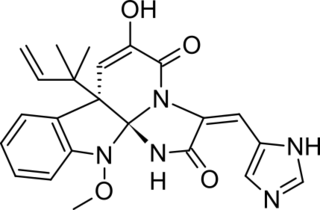
Meleagrin and its derivatives such as oxaline are bio-active benzylisoquinoline alkaloids made by various species of Penicillium fungi. It is similar to other fungal alkaloids, such as Roquefortine C, which is made as an intermediate in the same biosynthetic pathway.

Dideoxyverticillin A, also known as (+)-11,11′-dideoxyverticillin A, is a complex epipolythiodioxopiperazine initially isolated from the marine fungus Penicillium sp. in 1999. It has also been found in the marine fungus Bionectriaceae, and belongs to a class of naturally occurring 2,5-diketopiperazines.

Camalexin (3-thiazol-2-yl-indole) is a simple indole alkaloid found in the plant Arabidopsis thaliana and other crucifers. The secondary metabolite functions as a phytoalexin to deter bacterial and fungal pathogens.
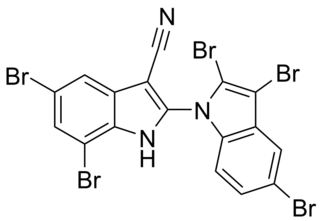
Aetokthonotoxin (AETX), colloquially known as eagle toxin, is a chemical compound that was identified in 2021 as the cyanobacterial neurotoxin causing vacuolar myelinopathy (VM) in eagles in North America. As the biosynthesis of aetokthonotoxin depends on the availability of bromide in freshwater systems and requires an interplay between the toxin-producing cyanobacterium Aetokthonos hydrillicola and the host plant it epiphytically grows on, it took more than 25 years to identify aetokthonotoxin as the VM-inducing toxin after the disease has first been diagnosed in bald eagles in 1994. The toxin cascades through the food-chain: Among other animals, it affects fish and waterfowl such as coots or ducks which feed on hydrilla colonized with the cyanobacterium. Aetokthonotoxin is transmitted to raptors, such as the bald eagle, that prey on these affected animals. The total synthesis of AETX has been achieved in 2021, the enzymatic functions of the 5 enzymes involved in AETX biosynthesis were described in 2022.