
Boiling or ebullition is the rapid phase transition from liquid to gas or vapour; the reverse of boiling is condensation. Boiling occurs when a liquid is heated to its boiling point, so that the vapour pressure of the liquid is equal to the pressure exerted on the liquid by the surrounding atmosphere. Boiling and evaporation are the two main forms of liquid vapourization.

Humidity is the concentration of water vapor present in the air. Water vapor, the gaseous state of water, is generally invisible to the human eye. Humidity indicates the likelihood for precipitation, dew, or fog to be present.

An aerosol is a suspension of fine solid particles or liquid droplets in air or another gas. Aerosols can be generated from natural or human causes. The term aerosol commonly refers to the mixture of particulates in air, and not to the particulate matter alone. Examples of natural aerosols are fog, mist or dust. Examples of human caused aerosols include particulate air pollutants, mist from the discharge at hydroelectric dams, irrigation mist, perfume from atomizers, smoke, dust, sprayed pesticides, and medical treatments for respiratory illnesses.
Water vapor, water vapour or aqueous vapor is the gaseous phase of water. It is one state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from the sublimation of ice. Water vapor is transparent, like most constituents of the atmosphere. Under typical atmospheric conditions, water vapor is continuously generated by evaporation and removed by condensation. It is less dense than most of the other constituents of air and triggers convection currents that can lead to clouds and fog.

Spray drying is a method of forming a dry powder from a liquid or slurry by rapidly drying with a hot gas. This is the preferred method of drying of many thermally-sensitive materials such as foods and pharmaceuticals, or materials which may require extremely consistent, fine particle size. Air is most commonly used as the heated drying medium; however, nitrogen may be used if the liquid is flammable or if the product is oxygen-sensitive.

Mist is a phenomenon caused by small droplets of water suspended in the cold air, usually by condensation. Physically, it is an example of a dispersion. It is most commonly seen where water vapor in warm, moist air meets sudden cooling, such as in exhaled air in the winter, or when throwing water onto the hot stove of a sauna. It can be created artificially with aerosol canisters if the humidity and temperature conditions are right. It can also occur as part of natural weather, when humid air cools rapidly, notably when the air comes into contact with surfaces that are much cooler than the air.

A cloud chamber, also known as a Wilson chamber, is a particle detector used for visualizing the passage of ionizing radiation.

Cloud condensation nuclei (CCNs), also known as cloud seeds, are small particles typically 0.2 μm, or one hundredth the size of a cloud droplet. CCNs are a unique subset of aerosols in the atmosphere on which water vapour condenses. This can affect the radiative properties of clouds and the overall atmosphere. Water vapour requires a non-gaseous surface to make the transition to a liquid; this process is called condensation.

Cloud physics is the study of the physical processes that lead to the formation, growth and precipitation of atmospheric clouds. These aerosols are found in the troposphere, stratosphere, and mesosphere, which collectively make up the greatest part of the homosphere. Clouds consist of microscopic droplets of liquid water, tiny crystals of ice, or both, along with microscopic particles of dust, smoke, or other matter, known as condensation nuclei. Cloud droplets initially form by the condensation of water vapor onto condensation nuclei when the supersaturation of air exceeds a critical value according to Köhler theory. Cloud condensation nuclei are necessary for cloud droplets formation because of the Kelvin effect, which describes the change in saturation vapor pressure due to a curved surface. At small radii, the amount of supersaturation needed for condensation to occur is so large, that it does not happen naturally. Raoult's law describes how the vapor pressure is dependent on the amount of solute in a solution. At high concentrations, when the cloud droplets are small, the supersaturation required is smaller than without the presence of a nucleus.

Crystallization is the process by which solids form, where the atoms or molecules are highly organized into a structure known as a crystal. Some ways by which crystals form are precipitating from a solution, freezing, or more rarely deposition directly from a gas. Attributes of the resulting crystal depend largely on factors such as temperature, air pressure, cooling rate, and in the case of liquid crystals, time of fluid evaporation.

In thermodynamics, nucleation is the first step in the formation of either a new thermodynamic phase or structure via self-assembly or self-organization within a substance or mixture. Nucleation is typically defined to be the process that determines how long an observer has to wait before the new phase or self-organized structure appears. For example, if a volume of water is cooled significantly below 0 °C, it will tend to freeze into ice, but volumes of water cooled only a few degrees below 0 °C often stay completely free of ice for long periods (supercooling). At these conditions, nucleation of ice is either slow or does not occur at all. However, at lower temperatures nucleation is fast, and ice crystals appear after little or no delay.
The Wegener–Bergeron–Findeisen process, is a process of ice crystal growth that occurs in mixed phase clouds in regions where the ambient vapor pressure falls between the saturation vapor pressure over water and the lower saturation vapor pressure over ice. This is a subsaturated environment for liquid water but a supersaturated environment for ice resulting in rapid evaporation of liquid water and rapid ice crystal growth through vapor deposition. If the number density of ice is small compared to liquid water, the ice crystals can grow large enough to fall out of the cloud, melting into rain drops if lower level temperatures are warm enough.
In the physics of aerosols, deposition is the process by which aerosol particles collect or deposit themselves on solid surfaces, decreasing the concentration of the particles in the air. It can be divided into two sub-processes: dry and wet deposition. The rate of deposition, or the deposition velocity, is slowest for particles of an intermediate size. Mechanisms for deposition are most effective for either very small or very large particles. Very large particles will settle out quickly through sedimentation (settling) or impaction processes, while Brownian diffusion has the greatest influence on small particles. This is because very small particles coagulate in few hours until they achieve a diameter of 0.5 micrometres. At this size they no longer coagulate. This has a great influence in the amount of PM-2.5 present in the air.
The Kelvin equation describes the change in vapour pressure due to a curved liquid–vapor interface, such as the surface of a droplet. The vapor pressure at a convex curved surface is higher than that at a flat surface. The Kelvin equation is dependent upon thermodynamic principles and does not allude to special properties of materials. It is also used for determination of pore size distribution of a porous medium using adsorption porosimetry. The equation is named in honor of William Thomson, also known as Lord Kelvin.
The Stefan flow, occasionally called Stefan's flow, is a transport phenomenon concerning the movement of a chemical species by a flowing fluid that is induced to flow by the production or removal of the species at an interface. Any process that adds the species of interest to or removes it from the flowing fluid may cause the Stefan flow, but the most common processes include evaporation, condensation, chemical reaction, sublimation, ablation, adsorption, absorption, and desorption. It was named after the Slovenian physicist, mathematician, and poet Josef Stefan for his early work on calculating evaporation rates.
The term wet scrubber describes a variety of devices that remove pollutants from a furnace flue gas or from other gas streams. In a wet scrubber, the polluted gas stream is brought into contact with the scrubbing liquid, by spraying it with the liquid, by forcing it through a pool of liquid, or by some other contact method, so as to remove the pollutants.

The vapor–liquid–solid method (VLS) is a mechanism for the growth of one-dimensional structures, such as nanowires, from chemical vapor deposition. The growth of a crystal through direct adsorption of a gas phase on to a solid surface is generally very slow. The VLS mechanism circumvents this by introducing a catalytic liquid alloy phase which can rapidly adsorb a vapor to supersaturation levels, and from which crystal growth can subsequently occur from nucleated seeds at the liquid–solid interface. The physical characteristics of nanowires grown in this manner depend, in a controllable way, upon the size and physical properties of the liquid alloy.
A scanning mobility particle sizer (SMPS) is an analytical instrument that measures the size and number concentration of aerosol particles with diameters from 2.5 nm to 1000 nm. They employ a continuous, fast-scanning technique to provide high-resolution measurements.
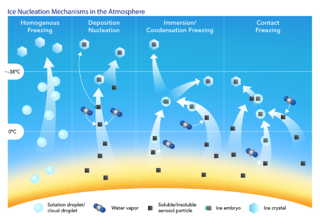
An ice nucleus, also known as an ice nucleating particle (INP), is a particle which acts as the nucleus for the formation of an ice crystal in the atmosphere.
In dropwise condensation the condensate liquid collects in the form of countless droplets of varying diameters on the condensing surface, instead of forming a continuous film, and does not wet the solid cooling surface. The droplets develop at points of surface imperfections, called nucleation sites, and grow in size as more vapour condenses on its exposed surface. When the size of droplets is large there comes a time the droplet breakaway from the surface and knock off other droplets and carries it downstream. The moving droplet devours the droplets of smaller size. Dropwise condensation is one of the most effective mechanism of heat transfer and extremely large heat transfer coefficients can be achieved with this mechanism. In dropwise condensation, there is no liquid film to resist heat transfer, and as a result heat transfer coefficients can be achieved more than 10 times larger than those associated with film condensation, although 3-5 times is more common. Heat transfer coefficients are large so designers can achieve a specified heat transfer rate with a smaller surface area and thus a smaller and less expensive condenser.













