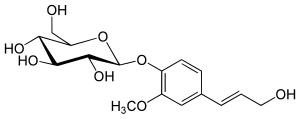
A glucoside is a glycoside that is chemically derived from glucose. Glucosides are common in plants, but rare in animals. Glucose is produced when a glucoside is hydrolysed by purely chemical means, or decomposed by fermentation or enzymes.

Angelica is a genus of about 90 species of tall biennial and perennial herbs in the family Apiaceae, native to temperate and subarctic regions of the Northern Hemisphere, reaching as far north as Iceland, Lapland, and Greenland. They grow to 1–3 m tall, with large bipinnate leaves and large compound umbels of white or greenish-white flowers. Found mainly in China, its main use was for medicine. It shows variations in fruit anatomy, leaf morphology, and subterranean structures. The genes are extremely polymorphic.

Angelica archangelica, commonly known as angelica, garden angelica, wild celery, and Norwegian angelica, is a biennial plant from the family Apiaceae, a subspecies of which is cultivated for its sweetly scented edible stems and roots. Like several other species in Apiaceae, its appearance is similar to several poisonous species, and should not be consumed unless it has been identified with absolute certainty. Synonyms include Archangelica officinalisHoffm. and Angelica officinalisMoench.
Angelica is a genus of herbs, especially the cultivated species Angelica archangelica

Imperatorin is a furocoumarin and a phytochemical that has been isolated from Urena lobata L. (Malvaceae), Angelica archangelica, Angelica dahurica, Glehnia littoralis, Saposhnikovia divaricata, Cnidium monnieri, Incarvillea younghusbandii, and Zanthoxylum americanum mill. It is biosynthesized from umbelliferone, a coumarin derivative.

Coniferyl alcohol is an organic compound with the formula HO(CH3O)C6H3CH=CHCH2OH. A colourless or white solid, it is one of the monolignols, produced via the phenylpropanoid biochemical pathway. When copolymerized with related aromatic compounds, coniferyl alcohol forms lignin or lignans. Coniferin is a glucoside of coniferyl alcohol. Coniferyl alcohol is an intermediate in biosynthesis of eugenol and of stilbenoids and coumarin. Gum benzoin contains significant amount of coniferyl alcohol and its esters. It is found in both gymnosperm and angiosperm plants. Sinapyl alcohol and paracoumaryl alcohol, the other two lignin monomers, are found in angiosperm plants and grasses.

Apterin is a furanocoumarin and the glucoside of vaginol. It has been isolated from the root of plants in the family Apiaceae such as members of the genus Angelica, including the garden angelica and Zizia aptera.

Swedish bitters, also called Swedish tincture, is a bitter and a traditional herbal tonic, the use of which dates back to the 15th century.

Meum is a monotypic genus in the family Apiaceae. Its only species is Meum athamanticum, a glabrous, highly aromatic, perennial plant. Common names in the UK include baldmoney, meu or meum, and spignel.
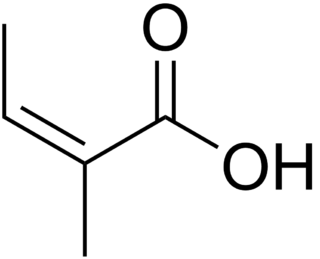
Angelic acid is a monocarboxylic unsaturated organic acid, mostly found in the plants of the family Apiaceae. It is a volatile solid with a biting taste and pungent sour odor. It is the cis isomer of 2-methyl-2-butenoic acid, which easily converts to the trans isomer, tiglic acid, upon heating or reaction with inorganic acids. The reverse transformation occurs much less readily. The salts and esters of angelic acid are called angelates. Angelic acid esters are the active components of herbal medicine used against a wide range of various health disturbances including pains, fever, gout, heartburn, etc.

Arådalen is a valley immediately west of the mountain Hundshögen in southern Oviksfjällen, Oviken parish, Berg's Municipality, Jämtland, Sweden. It has been named after the watercourse Arån as from the valley is flowing southward.
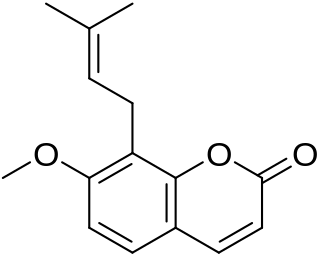
Osthol, or osthole, is a chemical compound which is a derivative of coumarin. It is found in a variety of plants including Cnidium monnieri, Angelica archangelica and Angelica pubescens.

Dasypolia templi, the brindled ochre, is a moth of the family Noctuidae. The species was first described by Carl Peter Thunberg in 1792. It is found in northern Europe up to central Siberia and more to the south in mountainous areas.

Simyra albovenosa, the reed dagger, is a moth of the family Noctuidae. It is found in most of Europe, then Turkey, Iran, Transcaucasus and into the east Palearctic.

Eupithecia lanceata is a moth of the family Geometridae. It is found from northern Europe to Anatolia.

Fadno is a reed instrument and domestic flute of the Sami people of Scandinavia, made from Angelica archangelica. The instrument features a reed and three to six fingerholes and appears to have no parallels among the surrounding Scandinavian peoples.

Agonopterix curvipunctosa is a moth of the family Depressariidae. It is found in most of Europe, except Ireland, Portugal, Finland, the Baltic region and the western and southern part of the Balkan Peninsula.

Epermenia chaerophyllella, also known as the garden lance-wing, is a moth of the family Epermeniidae first described by Johann August Ephraim Goeze in 1783. It is found in all of Europe and Asia Minor.
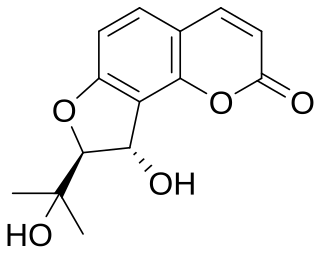
Vaginol is a chemical compound of the furanocoumarin class. Its glucoside is apterin.

Ligustilide is a natural chemical compound of the dihydrophthalide class. Ligustilide is found in the highest concentration in wild celeries. It has also been found in Angelica sinensis and a variety of other plants including Todaroa montana.