
Chymotrypsin (EC 3.4.21.1, chymotrypsins A and B, alpha-chymar ophth, avazyme, chymar, chymotest, enzeon, quimar, quimotrase, alpha-chymar, alpha-chymotrypsin A, alpha-chymotrypsin) is a digestive enzyme component of pancreatic juice acting in the duodenum, where it performs proteolysis, the breakdown of proteins and polypeptides. Chymotrypsin preferentially cleaves peptide amide bonds where the side chain of the amino acid N-terminal to the scissile amide bond (the P1 position) is a large hydrophobic amino acid (tyrosine, tryptophan, and phenylalanine). These amino acids contain an aromatic ring in their side chain that fits into a hydrophobic pocket (the S1 position) of the enzyme. It is activated in the presence of trypsin. The hydrophobic and shape complementarity between the peptide substrate P1 side chain and the enzyme S1 binding cavity accounts for the substrate specificity of this enzyme. Chymotrypsin also hydrolyzes other amide bonds in peptides at slower rates, particularly those containing leucine and methionine at the P1 position.

Conidae, with the current common name of "cone snails", is a taxonomic family of predatory sea snails, marine gastropod molluscs in the superfamily Conoidea.

Proteinogenic amino acids are amino acids that are incorporated biosynthetically into proteins during translation. The word "proteinogenic" means "protein creating". Throughout known life, there are 22 genetically encoded (proteinogenic) amino acids, 20 in the standard genetic code and an additional 2 that can be incorporated by special translation mechanisms.

Ziconotide, also called intrathecal ziconotide (ITZ) because of its administration route, is an atypical analgesic agent for the amelioration of severe and chronic pain. Derived from Conus magus, a cone snail, it is the synthetic form of an ω-conotoxin peptide. It is 1,000 times as powerful as morphine.

A conotoxin is one of a group of neurotoxic peptides isolated from the venom of the marine cone snail, genus Conus.

Tropidolaemus wagleri is a species of venomous snake, a pitviper in the subfamily Crotalinae of the family Viperidae. The species is endemic to Southeast Asia. There are no subspecies that are recognized as being valid. It is sometimes referred to as the temple viper because of its abundance around the Temple of the Azure Cloud in Malaysia.

Cone snails, cone shells, or cones are a large group of small- to large-sized extremely venomous predatory sea snails, marine gastropod molluscs.

Lourdes J. Cruz is a Filipino biochemist whose research has contributed to the understanding of the biochemistry of toxic peptides from the venom of fish-hunting Conus marine snails. Throughout the Philippines, she is known as the Sea Snail Venom Specialist. The characterization of over 50 biologically active peptides from the snail's venom had been made possible, in part, by her studies. Scientific findings regarding the peptides found in snails have applications in diagnostic tools for cancers and the development of drugs for the treatment of neurological disorders. She has also contributed to the development of conotoxins as tools for examining the activity of the human brain. Her contributions to science have earned her several awards and acknowledgements including being named a National Scientist of the Philippines in 2006.

Conus bullatus, common name the bubble cone, is a species of sea snail, a marine gastropod mollusk in the family Conidae, the cone snails and their allies.
Conus parius, common name the Parian cone, is a species of sea snail, a marine gastropod mollusk in the family Conidae, the cone snails and their allies.

Conus regius, common name the "crown cone", is a species of sea snail, a marine gastropod mollusk in the family Conidae, the cone snails and their allies.

Conus victoriae, common name the Queen Victoria cone, is a species of sea snail, a marine gastropod mollusk in the family Conidae, the cone snails and their allies.

WALP peptides are a class of synthesized, membrane-spanning α-helices composed of tryptophan (W), alanine (A), and leucine (L) amino acids. They are designed to study properties of proteins in lipid membranes such as orientation, extent of insertion, and hydrophobic mismatch.
BmKAEP is a neurotoxin from the venom of the Manchurian scorpion (Mesobuthus martensii). It is a β-toxin, which shift the activation voltage of sodium channels towards more negative potentials.
Conantokins are a small family of helical peptides that are derived from the venom of predatory marine snails of the genus Conus. Conantokins act as potent and specific antagonists of the N-methyl-D-aspartate receptor (NMDAR). They are the only naturally-derived peptides to do so. The subtypes of conantokins exhibit a surprising variability of selectivity across the NMDAR subunits, and are therefore uniquely useful in developing subunit-specific pharmacological probes.

Conus ventricosus, common name the Mediterranean cone, is a species of sea snail, a marine gastropod mollusk in the family Conidae, the cone snails and their allies.

ConoServer is a database of toxins that are expressed by the predatory sea snails in the family Conidae, the cone snails. These toxins are known as conotoxins or conopeptides. The toxins are of importance to medical research. A notable feature of these peptides is their high specificity and affinity towards human ion channels, receptors and transporters of the nervous system. This makes conopeptides an interesting resource for the physiological studies of neuroreceptors and promising drug leads.
Leconotide is an ω-conotoxin peptide isolated from the venom of Conus catus which is under investigation as an analgesic drug for the treatment of pain conditions.
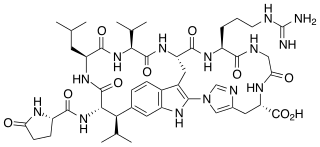
Moroidin is a biologically active compound found in the plants Dendrocnide moroides and Celosia argentea. It is a peptide composed of eight amino acids, with unusual leucine-tryptophan and tryptophan-histidine cross-links that form its two rings. Moroidin has been shown to be at least one of several bioactive compounds responsible for the painful sting of the Dendrocnide moroides plant. It also has demonstrated anti-mitotic properties, specifically by inhibition of tubulin polymerization. Anti-mitotic activity gives moroidin potential as a chemotherapy drug, and this property combined with its unusual chemical structure has made it a target for organic synthesis.
CNF-Sr3, also known as conorfamide-Sr3, is a toxin derived from the venom duct of Conus spurius. CNF-Sr3 is an inhibitor of the Shaker channel, a subtype of the voltage-gated potassium channels.















