Related Research Articles
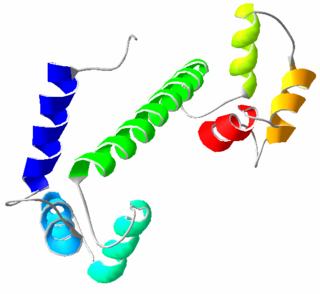
Calmodulin (CaM) (an abbreviation for calcium-modulated protein) is a multifunctional intermediate calcium-binding messenger protein expressed in all eukaryotic cells. It is an intracellular target of the secondary messenger Ca2+, and the binding of Ca2+ is required for the activation of calmodulin. Once bound to Ca2+, calmodulin acts as part of a calcium signal transduction pathway by modifying its interactions with various target proteins such as kinases or phosphatases.
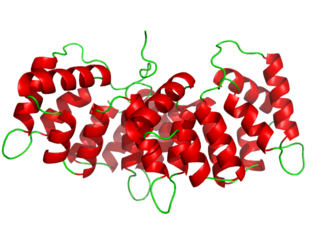
Annexin is a common name for a group of cellular proteins. They are mostly found in eukaryotic organisms.

Phosphatidylinositol 4,5-bisphosphate or PtdIns(4,5)P2, also known simply as PIP2 or PI(4,5)P2, is a minor phospholipid component of cell membranes. PtdIns(4,5)P2 is enriched at the plasma membrane where it is a substrate for a number of important signaling proteins.

Synaptotagmins (SYTs) constitute a family of membrane-trafficking proteins that are characterized by an N-terminal transmembrane region (TMR), a variable linker, and two C-terminal C2 domains - C2A and C2B. There are 17 isoforms in the mammalian synaptotagmin family. There are several C2-domain containing protein families that are related to synaptotagmins, including transmembrane (Ferlins, Extended-Synaptotagmin (E-Syt) membrane proteins, and MCTPs) and soluble (RIMS1 and RIMS2, UNC13D, synaptotagmin-related proteins and B/K) proteins. The family includes synaptotagmin 1, a Ca2+ sensor in the membrane of the pre-synaptic axon terminal, coded by gene SYT1.

A C2 domain is a protein structural domain involved in targeting proteins to cell membranes. The typical version (PKC-C2) has a beta-sandwich composed of 8 β-strands that co-ordinates two or three calcium ions, which bind in a cavity formed by the first and final loops of the domain, on the membrane binding face. Many other C2 domain families don't have calcium binding activity.

Annexin A5 is a cellular protein in the annexin group. In flow cytometry, annexin V is commonly used to detect apoptotic cells by its ability to bind to phosphatidylserine, a marker of apoptosis when it is on the outer leaflet of the plasma membrane. The function of the protein is unknown; however, annexin A5 has been proposed to play a role in the inhibition of blood coagulation by competing for phosphatidylserine binding sites with prothrombin and also to inhibit the activity of phospholipase A1. These properties have been found by in vitro experiments.
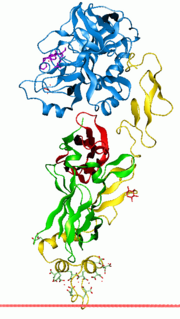
Vitamin K-dependent carboxylation/gamma-carboxyglutamic (GLA) domain is a protein domain that contains post-translational modifications of many glutamate residues by vitamin K-dependent carboxylation to form γ-carboxyglutamate (Gla). Proteins with this domain are known informally as Gla proteins. The Gla residues are responsible for the high-affinity binding of calcium ions.
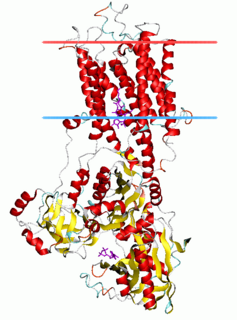
The P-type ATPases, also known as E1-E2 ATPases, are a large group of evolutionarily related ion and lipid pumps that are found in bacteria, archaea, and eukaryotes. P-type ATPases are α-helical bundle primary transporters named based upon their ability to catalyze auto- (or self-) phosphorylation (hence P) of a key conserved aspartate residue within the pump and their energy source, adenosine triphosphate (ATP). In addition, they all appear to interconvert between at least two different conformations, denoted by E1 and E2. P-type ATPases fall under the P-type ATPase (P-ATPase) Superfamily (TC# 3.A.3) which, as of early 2016, includes 20 different protein families.

Phospholipase C (PLC) is a class of membrane-associated enzymes that cleave phospholipids just before the phosphate group (see figure). It is most commonly taken to be synonymous with the human forms of this enzyme, which play an important role in eukaryotic cell physiology, in particular signal transduction pathways. There are thirteen kinds of mammalian phospholipase C that are classified into six isotypes (β, γ, δ, ε, ζ, η) according to structure. Each PLC has unique and overlapping controls over expression and subcellular distribution. Activators of each PLC vary, but typically include heterotrimeric G protein subunits, protein tyrosine kinases, small G proteins, Ca2+, and phospholipids.

Annexin A7 is a protein that in humans is encoded by the ANXA7 gene.

Copine-8 is a protein that in humans is encoded by the CPNE8 gene.

Calcium-binding mitochondrial carrier protein Aralar1 is a protein that in humans is encoded by the SLC25A12 gene. Aralar is an integral membrane protein located in the inner mitochondrial membrane. Its primary function as an antiporter is the transport of cytoplasmic glutamate with mitochondrial aspartate across the inner mitochondrial membrane, dependent on the binding of one calcium ion. Mutations in this gene cause early infantile epileptic encephalopathy 39 (EIEE39), symptomized by global hypomyelination of the central nervous system, refractory seizures, and neurodevelopmental impairment. This gene has connections to autism.

Copine-1 is a protein that in humans is encoded by the CPNE1 gene.

Copine-6 is a protein that in humans is encoded by the CPNE6 gene.

Copine-4 is a protein that in humans is encoded by the CPNE4 gene.

Syntaxins are a family of membrane integrated Q-SNARE proteins participating in exocytosis.
The Thiol-activated Cholesterol-dependent Cytolysin(CDC) family is a member of the MACPF superfamily. Cholesterol dependent cytolysins are a family of β-barrel pore-forming exotoxins that are secreted by gram-positive bacteria. CDCs are secreted as water-soluble monomers of 50-70 kDa, that when bound to the target cell, form a circular homo-oligomeric complex containing as many as 40 monomers. Through multiple conformational changes, the β-barrel transmembrane structure is formed and inserted into the target cell membrane. The presence of cholesterol in the target membrane is required for pore formation, though the presence of cholesterol is not required by all CDCs for binding. For example, Intermedilysin secreted by Streptococcus intermedius will bind only to target membranes containing a specific protein receptor, independent of the presence of cholesterol, but cholesterol is required by intermedilysin for pore formation. While the lipid environment of cholesterol in the membrane can affect toxin binding, the exact molecular mechanism that cholesterol regulates the cytolytic activity of the CDC is not fully understood.

Coiled-coil domain containing 22 is a protein that in humans is encoded by the CCDC22 gene.

Copine 3 is a protein that in humans is encoded by the CPNE3 gene.
Ferlins are an ancient protein family involved in vesicle fusion and membrane trafficking. Ferlins are distinguished by their multiple tandem C2 domains, and sometimes a FerA and a DysF domain. Mutations in ferlins can cause human diseases such as muscular dystrophy and deafness. Abnormalities in expression of myoferlin, a human ferlin protein, is also directly associated with higher mortality rate and tumor recurrence in several types of cancer, including pancreatic, colorectal, breast, cervical, stomach, ovarian, cervical, thyroid, endometrial, and oropharyngeal squamous cell carcinoma. In other animals, ferlin mutations can cause infertility.
References
- ↑ "copine family". UniProtKB. Retrieved 28 March 2013.
- ↑ Damer, Cynthia K; Marina Bayeva; Emily S Hahn; Javier Rivera; Catherine I Socec (2005). "Copine A, a calcium-dependent membrane-binding protein, transiently localizes to the plasma membrane and intracellular vacuoles in Dictyostelium". BMC Cell Biology. 6: 46. doi:10.1186/1471-2121-6-46. PMC 1327671 . PMID 16343335.
- 1 2 Tomsig, Jose Luis; Creutz, Carl E. (2000-12-01). "Biochemical Characterization of Copine: A Ubiquitous Ca2+-Dependent, Phospholipid-Binding Protein". Biochemistry. 39 (51): 16163–16175. doi:10.1021/bi0019949. ISSN 0006-2960. PMID 11123945.
- ↑ Creutz CE, Tomsig JL, Snyder SL, Gautier MC, Skouri F, Beisson J, Cohen J (Feb 1998). "The copines, a novel class of C2 domain-containing, calcium-dependent, phospholipid-binding proteins conserved from Paramecium to humans". J Biol Chem. 273 (3): 1393–402. doi: 10.1074/jbc.273.3.1393 . PMID 9430674.
| This protein-related article is a stub. You can help Wikipedia by expanding it. |