This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.

Hereditary coproporphyria (HCP) is a disorder of heme biosynthesis, classified as an acute hepatic porphyria. HCP is caused by a deficiency of the enzyme coproporphyrinogen oxidase, coded for by the CPOX gene, and is inherited in an autosomal dominant fashion, although homozygous individuals have been identified. Unlike acute intermittent porphyria, individuals with HCP can present with cutaneous findings similar to those found in porphyria cutanea tarda in addition to the acute attacks of abdominal pain, vomiting and neurological dysfunction characteristic of acute porphyrias. Like other porphyrias, attacks of HCP can be induced by certain drugs, environmental stressors or diet changes. Biochemical and molecular testing can be used to narrow down the diagnosis of a porphyria and identify the specific genetic defect. Overall, porphyrias are rare diseases. The combined incidence for all forms of the disease has been estimated at 1:20,000. The exact incidence of HCP is difficult to determine, due to its reduced penetrance.
Porphyria cutanea tarda (PCT) is the most common subtype of porphyria. The disease is named because it is a porphyria that often presents with skin manifestations later in life. The disorder results from low levels of the enzyme responsible for the fifth step in heme production. Heme is a vital molecule for all of the body's organs. It is a component of hemoglobin, the molecule that carries oxygen in the blood.
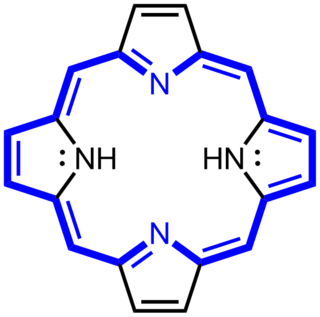
Gunther disease, also known as congenital erythropoietic porphyria (CEP), uroporphyrinogen III synthase deficiency and UROS deficiency, is a congenital form of erythropoietic porphyria. The word porphyria originated from the Greek word porphura. Porphura actually means "purple pigment", which, in suggestion, the color that the body fluid changes when a person has Gunther's disease. It is a rare, autosomal recessive metabolic disorder affecting heme, caused by deficiency of the enzyme uroporphyrinogen cosynthetase. It is extremely rare, with a prevalence estimated at 1 in 1,000,000 or less. There have been times that prior to birth of a fetus, Gunther's disease has been shown to lead to anemia. In milder cases patients have not presented any symptoms until they have reached adulthood. In Gunther's disease, porphyrins are accumulated in the teeth and bones and an increased amount are seen in the plasma, bone marrow, feces, red blood cells, and urine.

Uroporphyrinogen III decarboxylase is an enzyme that in humans is encoded by the UROD gene.

Uroporphyrinogen III synthase EC 4.2.1.75 is an enzyme involved in the metabolism of the cyclic tetrapyrrole compound porphyrin. It is involved in the conversion of hydroxymethyl bilane into uroporphyrinogen III. This enzyme catalyses the inversion of the final pyrrole unit of the linear tetrapyrrole molecule, linking it to the first pyrrole unit, thereby generating a large macrocyclic structure, uroporphyrinogen III. The enzyme folds into two alpha/beta domains connected by a beta-ladder, the active site being located between the two domains.
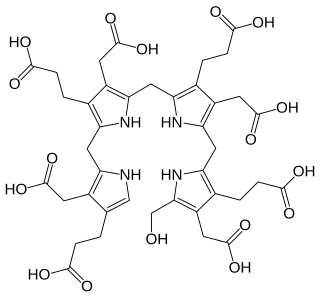
Coproporphyrinogen-III oxidase, mitochondrial is an enzyme that in humans is encoded by the CPOX gene. A genetic defect in the enzyme results in a reduced production of heme in animals. The medical condition associated with this enzyme defect is called hereditary coproporphyria.

Protoporphyrinogen IX is an organic chemical compound which is produced along the synthesis of porphyrins, a class of critical biochemicals that include hemoglobin and chlorophyll. It is a direct precursor of protoporphyrin IX.

Hydroxymethylbilane, also known as preuroporphyrinogen, is an organic compound that occurs in living organisms during the synthesis of porphyrins, a group of critical substances that include hemoglobin, myoglobin, and chorophyll. The name is often abbreviated as HMB.

Hepatoerythropoietic porphyria is a very rare form of hepatic porphyria caused by a disorder in both genes which code Uroporphyrinogen III decarboxylase (UROD).
In enzymology, a coproporphyrinogen dehydrogenase (EC 1.3.99.22) is an enzyme that catalyzes the chemical reaction
The molecular formula C36H44N4O8 (molar mass: 660.75 g/mol, exact mass: 660.315914) may refer to:
Uroporphyrinogen-III C-methyltransferase is an enzyme with systematic name S-adenosyl-L-methionine:uroporphyrinogen-III C-methyltransferase. This enzyme catalyses the following chemical reaction
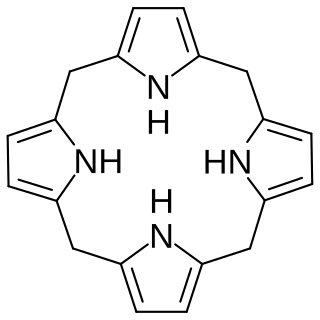
In biochemistry a porphyrinogen is a member of a class of naturally occurring compounds with a tetrapyrrole core, a macrocycle of four pyrrole rings connected by four methylene bridges. They can be viewed as derived from the parent compound hexahydroporphine by the substitution of various functional groups for hydrogen atoms in the outermost (20-carbon) ring.
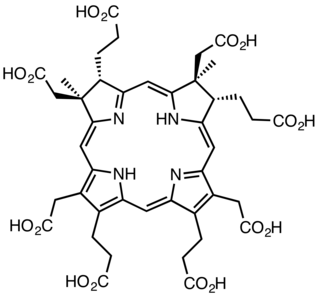
Sirohydrochlorin (SHC) is a macrocyclic metabolic intermediate in the biosynthesis of corrin, precursor to the tetrapyrrole pigment and cofactor F430. It is a yellow compound. Its biosynthetic precursor is dihydrosirohydrochlorin, which in turn is derived from uroporphyrinogen III. It is also the precursor to the siroheme, the prosthetic group in a sulfite reductase.