External links
- Uroporphyrinogens at the US National Library of Medicine Medical Subject Headings (MeSH)
Types of tetrapyrroles | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bilanes (Linear) |
| ||||||||||||||||||||
| Macrocycle |
| ||||||||||||||||||||
| | This biochemistry article is a stub. You can help Wikipedia by expanding it. |
Uroporphyrinogens are cyclic tetrapyrroles with four propionic acid groups ("P" groups) and four acetic acid groups ("A" groups).
There are four forms, which vary based upon the arrangements of the "P" and "A" groups (in clockwise order):

Heme, or haem, is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver.
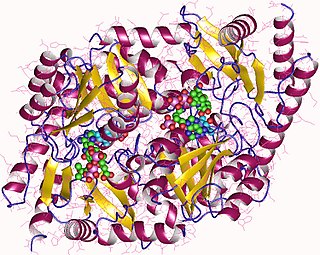
Aminolevulinic acid synthase (ALA synthase, ALAS, or delta-aminolevulinic acid synthase) is an enzyme (EC 2.3.1.37) that catalyzes the synthesis of δ-aminolevulinic acid (ALA) the first common precursor in the biosynthesis of all tetrapyrroles such as hemes, cobalamins and chlorophylls. The reaction is as follows:
Tetrapyrroles are a class of chemical compounds that contain four pyrrole or pyrrole-like rings. The pyrrole/pyrrole derivatives are linked by, in either a linear or a cyclic fashion. Pyrroles are a five-atom ring with four carbon atoms and one nitrogen atom. Tetrapyrroles are common cofactors in biochemistry and their biosynthesis and degradation feature prominently in the chemistry of life.

Uroporphyrinogen III decarboxylase is an enzyme that in humans is encoded by the UROD gene.

Uroporphyrinogen III synthase EC 4.2.1.75 is an enzyme involved in the metabolism of the cyclic tetrapyrrole compound porphyrin. It is involved in the conversion of hydroxymethyl bilane into uroporphyrinogen III. This enzyme catalyses the inversion of the final pyrrole unit of the linear tetrapyrrole molecule, linking it to the first pyrrole unit, thereby generating a large macrocyclic structure, uroporphyrinogen III. The enzyme folds into two alpha/beta domains connected by a beta-ladder, the active site being located between the two domains.
Coproporphyrinogens are tetrapyrroles with four propionic acid groups and an equal number of substituted methyls.

Coproporphyrinogen III is a metabolic intermediate in the biosynthesis of many compounds that are critical for living organisms, such as hemoglobin and chlorophyll. It is a colorless solid.

Uroporphyrinogen III is a tetrapyrrole, the first macrocyclic intermediate in the biosynthesis of heme, chlorophyll, vitamin B12, and siroheme. It is a colorless compound, like other porphyrinogens.
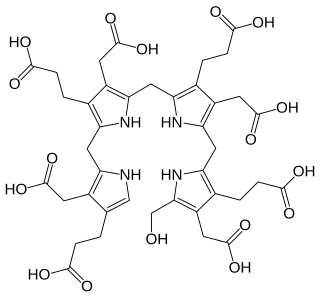
Hydroxymethylbilane, also known as preuroporphyrinogen, is an organic compound that occurs in living organisms during the synthesis of porphyrins, a group of critical substances that include haemoglobin, myoglobin, and chlorophyll. The name is often abbreviated as HMB.
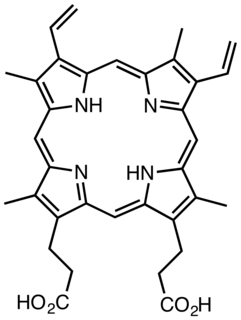
Protoporphyrin IX is an organic compound, classified as a porphyrin, that plays an important role in living organisms as a precursor to other critical compounds like heme (hemoglobin) and chlorophyll. It is a deeply colored solid that is not soluble in water. The name is often abbreviated as PPIX.

Uroporphyrinogen I is an isomer of uroporphyrinogen III, a metabolic intermediate in the biosynthesis of heme. A type of porphyria is caused by production of uroporphyrinogen I instead of III.

Coproporphyrinogen I is an isomer of coproporphyrinogen III, a metabolic intermediate in the normal biosynthesis of heme. The compound is not normally produced by the human body; its production and accumulation causes a type of porphyria.
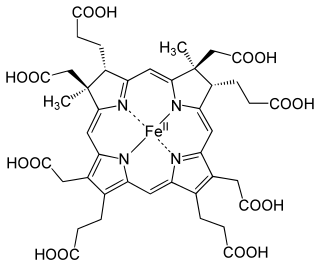
In enzymology, a sirohydrochlorin ferrochelatase (EC 4.99.1.4) is an enzyme that catalyzes the chemical reaction

Siroheme is a heme-like prosthetic group at the active sites of some enzymes to accomplish the six-electron reduction of sulfur and nitrogen. It is a cofactor at the active site of sulfite reductase, which plays a major role in sulfur assimilation pathway, converting sulfite into sulfide, which can be incorporated into the organic compound homocysteine.

Cobalamin biosynthesis is the process by which bacteria and archea make cobalamin, vitamin B12. Many steps are involved in converting aminolevulinic acid via uroporphyrinogen III and adenosylcobyric acid to the final forms in which it is used by enzymes in both the producing organisms and other species, including humans who acquire it through their diet.
In molecular biology, sirohaem synthase (CysG) is a multi-functional enzyme with S-adenosyl-L-methionine (SAM)-dependent bismethyltransferase, dehydrogenase and ferrochelatase activities. Bacterial sulphur metabolism depends on the iron-containing porphinoid sirohaem. CysG synthesizes sirohaem from uroporphyrinogen III via reactions which encompass two branchpoint intermediates in tetrapyrrole biosynthesis, diverting flux first from protoporphyrin IX biosynthesis and then from cobalamin biosynthesis. CysG is a dimer. Its dimerisation region is 74 amino acids long, and acts to hold the two structurally similar protomers held together asymmetrically through a number of salt-bridges across complementary residues within the dimerisation region. CysG dimerisation produces a series of active sites, accounting for CysG's multi-functionality, catalysing four diverse reactions:
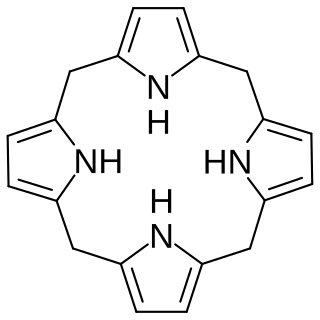
In biochemistry a porphyrinogen is a member of a class of naturally occurring compounds with a tetrapyrrole core, a macrocycle of four pyrrole rings connected by four methylene bridges. They can be viewed as derived from the parent compound hexahydroporphine by the substitution of various functional groups for hydrogen atoms in the outermost (20-carbon) ring.

Chlorophyllide a and Chlorophyllide b are the biosynthetic precursors of chlorophyll a and chlorophyll b respectively. Their propionic acid groups are converted to phytyl esters by the enzyme chlorophyll synthase in the final step of the pathway. Thus the main interest in these chemical compounds has been in the study of chlorophyll biosynthesis in plants, algae and cyanobacteria. Chlorophyllide a is also an intermediate in the biosynthesis of bacteriochlorophylls.
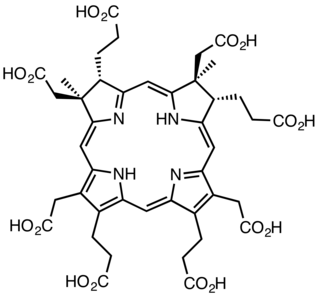
Sirohydrochlorin is a tetrapyrrole macrocyclic metabolic intermediate in the biosynthesis of sirohaem, the iron-containing prosthetic group in sulfite reductase enzymes. It is also the biosynthetic precursor to cofactor F430, an enzyme which catalyzes the release of methane in the final step of methanogenesis.

Dihydrosirohydrochlorin is one of several naturally occurring tetrapyrrole macrocyclic metabolic intermediates in the biosynthesis of vitamin B12 (cobalamin). Its oxidised form, sirohydrochlorin, is precursor to sirohaem, the iron-containing prosthetic group in sulfite reductase enzymes. Further biosynthetic transformations convert sirohydrochlorin to cofactor F430 for an enzyme which catalyzes the release of methane in the final step of methanogenesis.