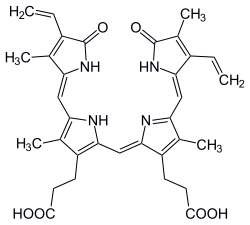
(3E)-phycoerythrobilin | |
| Names | |
|---|---|
| IUPAC name (2R,3E,16R)-18-ethenyl-3-ethylidene-1,2,3,15,16,19,22,24-octahydro-2,7,13,17-tetramethyl-1,19-dioxo-21H-biline-8,12-dipropanoic Acid | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| MeSH | phycoerythrobilin |
PubChem CID | |
| |
| |
| Properties | |
| C33H38N4O6 | |
| Molar mass | 586.689 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Phycoerythrobilin is a red phycobilin, i.e. an open tetrapyrrole chromophore [1] found in cyanobacteria and in the chloroplasts of red algae, glaucophytes and some cryptomonads. Phycoerythrobilin is present in the phycobiliprotein phycoerythrin, of which it is the terminal acceptor of energy. The amount of phycoerythrobilin in phycoerythrins varies a lot, depending on the considered organism. In some Rhodophytes and oceanic cyanobacteria, phycoerythrobilin is also present in the phycocyanin, then termed R-phycocyanin. Like all phycobilins, phycoerythrobilin is covalently linked to these phycobiliproteins by a thioether bond.