
In organic chemistry, an acetal is a functional group with the connectivity R2C(OR')2. Here, the R groups can be organic fragments or hydrogen, while the R' groups must be organic fragments not hydrogen. The two R' groups can be equivalent to each other or not. Acetals are formed from and convertible to aldehydes or ketones and have the same oxidation state at the central carbon, but have substantially different chemical stability and reactivity as compared to the analogous carbonyl compounds. The central carbon atom has four bonds to it, and is therefore saturated and has tetrahedral geometry.
Perfume is a mixture of fragrant essential oils or aroma compounds (fragrances), fixatives and solvents, usually in liquid form, used to give the human body, animals, food, objects, and living-spaces an agreeable scent. Perfumes can be defined as substances that emit and diffuse a pleasant and fragrant odor. They consist of manmade mixtures of aromatic chemicals and essential oils. The 1939 Nobel Laureate for Chemistry, Leopold Ružička stated in 1945 that "right from the earliest days of scientific chemistry up to the present time, perfumes have substantially contributed to the development of organic chemistry as regards methods, systematic classification, and theory."

Musk is a class of aromatic substances commonly used as base notes in perfumery. They include glandular secretions from animals such as the musk deer, numerous plants emitting similar fragrances, and artificial substances with similar odors. Musk was a name originally given to a substance with a strong odor obtained from a gland of the musk deer. The substance has been used as a popular perfume fixative since ancient times and is one of the most expensive animal products in the world. The name originates from the Late Greek μόσχος 'moskhos', from Persian mushk and Sanskrit मुष्क muṣka derived from Proto-Indo-European noun múh₂s meaning "mouse". The deer gland was thought to resemble a scrotum. It is applied to various plants and animals of similar smell and has come to encompass a wide variety of aromatic substances with similar odors, despite their often differing chemical structures and molecular shapes.

Play-Doh or also known as Play-Dough is a modeling compound for young children to make arts and crafts projects. The product was first manufactured in Cincinnati, Ohio, United States, as a wallpaper cleaner in the 1930s. Play-Doh was then reworked and marketed to Cincinnati schools in the mid-1950s. Play-Doh was demonstrated at an educational convention in 1956 and prominent department stores opened retail accounts.

1-Pentanol,, is an organic compound with the formula CH3CH2CH2CH2CH2OH and is classified as a primary alcohol. It is a colourless liquid with a distinctive aroma. It is one of 8 isomeric alcohols with the formula C5H11OH. It is used as a solvent, a biological drying agent and in the synthesis of some fragrance compounds. It is also a common component of fusel alcohols, the undesirable byproducts of alcoholic fermentation.

An aroma compound, also known as an odorant, aroma, fragrance or flavoring, is a chemical compound that has a smell or odor. For an individual chemical or class of chemical compounds to impart a smell or fragrance, it must be sufficiently volatile for transmission via the air to the olfactory system in the upper part of the nose. As examples, various fragrant fruits have diverse aroma compounds, particularly strawberries which are commercially cultivated to have appealing aromas, and contain several hundred aroma compounds.

Air fresheners are products designed to reduce unwanted odors in indoor spaces, or to introduce pleasant fragrances, or both. They typically emit fragrance to mask odors but may use other methods of action such as absorbing, bonding to, or chemically altering compounds in the air that produce smells, killing organisms that produce smells, or disrupting the sense of smell to reduce perception of unpleasant smells.
Fragrance oils, also known as aroma oils, aromatic oils, and flavor oils, are blended synthetic aroma compounds or natural essential oils that are diluted with a carrier like propylene glycol, vegetable oil, or mineral oil.
A fabric softener or fabric conditioner is a conditioner applied to laundry after it has been washed in a washing machine. A similar, more dilute preparation meant to be applied to dry fabric is known as a wrinkle releaser.

Chanel No. 5 is the first perfume launched by French couturier Gabrielle "Coco" Chanel in 1921. The scent formula for the fragrance was compounded by French-Russian chemist and perfumer Ernest Beaux. The design of its bottle has been an important part of the product's branding. Coco Chanel was the first face of the fragrance, appearing in the advertisement published by Harper's Bazaar in 1937.

The tribe Euglossini, in the subfamily Apinae, commonly known as orchid bees or euglossine bees, are the only group of corbiculate bees whose non-parasitic members do not all possess eusocial behavior.
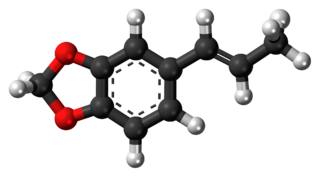
Isosafrole is an organic compound that is used in the fragrance industry. Structurally, the molecule is related to allylbenzene, a type of aromatic organic chemical. Its fragrance is reminiscent of anise or licorice. It is found in small amounts in various essential oils, but is most commonly obtained by isomerizing the plant oil safrole. It exists as two geometric isomers, cis-isosafrole and trans-isosafrole.

Citronellol, or dihydrogeraniol, is a natural acyclic monoterpenoid. Both enantiomers occur in nature. (+)-Citronellol, which is found in citronella oils, including Cymbopogon nardus (50%), is the more common isomer. (−)-Citronellol is widespread, but particularly abundant in the oils of rose (18–55%) and Pelargonium geraniums.

Farnesol is a natural 15-carbon organic compound which is an acyclic sesquiterpene alcohol. Under standard conditions, it is a colorless liquid. It is hydrophobic, and thus insoluble in water, but miscible with oils. As the pyrophosphate ester, farnesol is a precursor to many terpenes and terpenoids.
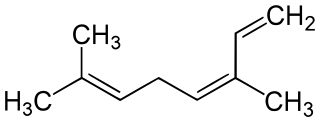
Ocimenes are a group of isomeric hydrocarbons. The ocimenes are monoterpenes found within a variety of plants and fruits. α-Ocimene and the two β-ocimenes differ in the position of the isolated double bond: it is terminal in the alpha isomer. α-Ocimene is cis-3,7-dimethyl-1,3,7-octatriene. β-Ocimene is trans-3,7-dimethyl-1,3,6-octatriene. β-Ocimene exists in two stereoisomeric forms, cis and trans, with respect to the central double bond. The ocimenes are often found naturally as mixtures of the various forms. The mixture, as well as the pure compounds, are oils with a pleasant odor. They are used in perfumery for their sweet herbal scent, and are believed to act as plant defense and have anti-fungal properties. Like the related acyclic terpene myrcene, ocimenes are unstable in air. Like other terpenes, the ocimenes are nearly insoluble in water, but soluble in common organic solvents.
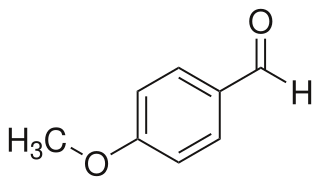
4-Anisaldehyde, or p-Anisaldehyde, is an organic compound with the formula CH3OC6H4CHO. The molecule consists of a benzene ring with a formyl and a methoxy group. It is a colorless liquid with a strong aroma. It provides sweet, floral and strong aniseed odor. Two isomers of 4-anisaldehyde are known, ortho-anisaldehyde and meta-anisaldehyde. They are less commonly encountered.

Symrise AG is a German chemicals company that is a major producer of flavours and fragrances with sales of €4.618 billion in 2022. Major competitors include Givaudan, Takasago International Corporation, International Flavors and Fragrances and Döhler. Symrise is a member of the European Flavour Association. In 2021, Symrise was ranked 4th by FoodTalks' Global Top 50 Food Flavours and Fragrances Companies list.

β-Pinene is a monoterpene, an organic compound found in plants. It is the less abundant of the two isomers of pinene, the other being α-pinene. It is a colorless liquid soluble in alcohol, but not water. It has a woody-green pine-like smell.

Notes in perfumery are descriptors of scents that can be sensed upon the application of a perfume. Notes are separated into three classes: top/head notes, middle/heart notes, and base/soul notes; which denote groups of scents which can be sensed with respect to the time after the application of a perfume. These notes are created with knowledge of the evaporation process and intended use of the perfume. The presence of one note may alter the perception of another—for instance, the presence of certain base or heart notes will alter the scent perceived when the top notes are strongest, and likewise the scent of base notes in the dry-down will often be altered depending on the smells of the heart notes.

Anisyl alcohol (4-methoxybenzyl alcohol) is an organic compound with the chemical formula CH3OC6H4CH2OH. It is a colorless liquid that is used as a fragrance and flavorant. It occurs naturally but is produced by reduction of the aldehyde or carboxylic acid. It reacts with hydrogen bromide to give 4-methoxylbenzyl bromide.

















