
Joseph Vissarionovich Stalin was a Soviet politician, political theorist and revolutionary who led the Soviet Union from 1924 until his death in 1953. He held power as General Secretary of the Communist Party of the Soviet Union (1922–1952) and Chairman of the Council of Ministers of the Soviet Union (1941–1953). Ideologically adhering to the Leninist interpretation of Marxism, he formalised these ideas as Marxism–Leninism, while his own policies are called Stalinism.

Sir Winston Leonard Spencer Churchill was a British statesman, soldier, and writer who served as Prime Minister of the United Kingdom twice, from 1940 to 1945 during the Second World War, and again from 1951 to 1955. Apart from two years between 1922 and 1924, he was a Member of Parliament (MP) from 1900 to 1964 and represented a total of five constituencies. Ideologically an economic liberal and imperialist, he was for most of his career a member of the Conservative Party, which he led from 1940 to 1955. He was a member of the Liberal Party from 1904 to 1924.
Melanin is a broad term for a group of natural pigments found in most organisms. The melanin pigments are produced in a specialized group of cells known as melanocytes.

The Gulf War was a 1990–1991 armed campaign waged by a 39-country military coalition in response to the Iraqi invasion of Kuwait. Spearheaded by the United States, the coalition's efforts against Iraq were carried out in two key phases: Operation Desert Shield, which marked the military buildup from August 1990 to January 1991; and Operation Desert Storm, which began with the aerial bombing campaign against Iraq on 17 January 1991 and came to a close with the American-led Liberation of Kuwait on 28 February 1991.

Hyperpigmentation is the darkening of an area of skin or nails caused by increased melanin.

Tyrosinase is an oxidase that is the rate-limiting enzyme for controlling the production of melanin. The enzyme is mainly involved in two distinct reactions of melanin synthesis otherwise known as the Raper Mason pathway. Firstly, the hydroxylation of a monophenol and secondly, the conversion of an o-diphenol to the corresponding o-quinone. o-Quinone undergoes several reactions to eventually form melanin. Tyrosinase is a copper-containing enzyme present in plant and animal tissues that catalyzes the production of melanin and other pigments from tyrosine by oxidation. It is found inside melanosomes which are synthesized in the skin melanocytes. In humans, the tyrosinase enzyme is encoded by the TYR gene.

Macrophage migration inhibitory factor (MIF), also known as glycosylation-inhibiting factor (GIF), L-dopachrome isomerase, or phenylpyruvate tautomerase is a protein that in humans is encoded by the MIF gene. MIF is an important regulator of innate immunity. The MIF protein superfamily also includes a second member with functionally related properties, the D-dopachrome tautomerase (D-DT). CD74 is a surface receptor for MIF.
In enzymology, a L-dopachrome isomerase is an enzyme that catalyzes the chemical reaction

G protein-coupled receptor 35 also known as GPR35 is a G protein-coupled receptor which in humans is encoded by the GPR35 gene. Heightened expression of GPR35 is found in immune and gastrointestinal tissues, including the crypts of Lieberkühn.
The enzyme D-dopachrome decarboxylase (EC 4.1.1.84) catalyzes the chemical reaction

Transcription factor SOX-10 is a protein that in humans is encoded by the SOX10 gene.
D-dopachrome decarboxylase is an enzyme that in humans is encoded by the DDT gene.

Indole-5,6-quinone is an indolequinone, a chemical compound found in the oxidative browning reaction of fruits like bananas where it is mediated by the tyrosinase type polyphenol oxidase from tyrosine and catecholamines leading to the formation of catechol melanin. Like many quinones it can undergo redox reactions via the corresponding 5,6-dihydroxyindole.

Macrophage migration inhibitory factor domain is an evolutionary conserved protein domain.

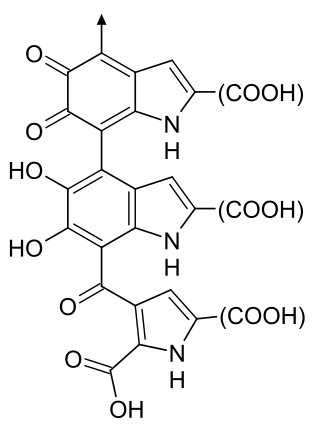
Dopachrome is a cyclization product of L-DOPA and is an intermediate in the biosynthesis of melanin. It may tautomerise to form DHICA.
The molecular formula C9H7NO4 (molar mass: 193.16 g/mol, exact mass: 193.0375 u) may refer to:

5,6-Dihydroxyindole is a chemical compound with the molecular formula C8H7NO2. It is an intermediate in the production of the biological pigment eumelanin. 5,6-Dihydroxyindole is biosynthesized from L-dopachrome in a reaction catalyzed by a tyrosinase enzyme and is further converted into indole-5,6-quinone. In humans, 5,6-dihydroxyindole is involved in the metabolic disorder hawkinsinuria.














