Signal transduction is the process by which a chemical or physical signal is transmitted through a cell as a series of molecular events. Proteins responsible for detecting stimuli are generally termed receptors, although in some cases the term sensor is used. The changes elicited by ligand binding in a receptor give rise to a biochemical cascade, which is a chain of biochemical events known as a signaling pathway.
A biochemical cascade, also known as a signaling cascade or signaling pathway, is a series of chemical reactions that occur within a biological cell when initiated by a stimulus. This stimulus, known as a first messenger, acts on a receptor that is transduced to the cell interior through second messengers which amplify the signal and transfer it to effector molecules, causing the cell to respond to the initial stimulus. Most biochemical cascades are series of events, in which one event triggers the next, in a linear fashion. At each step of the signaling cascade, various controlling factors are involved to regulate cellular actions, in order to respond effectively to cues about their changing internal and external environments.
Pattern recognition receptors (PRRs) play a crucial role in the proper function of the innate immune system. PRRs are germline-encoded host sensors, which detect molecules typical for the pathogens. They are proteins expressed mainly by cells of the innate immune system, such as dendritic cells, macrophages, monocytes, neutrophils, as well as by epithelial cells, to identify two classes of molecules: pathogen-associated molecular patterns (PAMPs), which are associated with microbial pathogens, and damage-associated molecular patterns (DAMPs), which are associated with components of host's cells that are released during cell damage or death. They are also called primitive pattern recognition receptors because they evolved before other parts of the immune system, particularly before adaptive immunity. PRRs also mediate the initiation of antigen-specific adaptive immune response and release of inflammatory cytokines.
The MAPK/ERK pathway is a chain of proteins in the cell that communicates a signal from a receptor on the surface of the cell to the DNA in the nucleus of the cell.

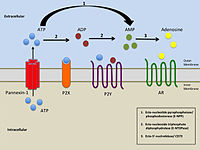
Purinergic receptors, also known as purinoceptors, are a family of plasma membrane molecules that are found in almost all mammalian tissues. Within the field of purinergic signalling, these receptors have been implicated in learning and memory, locomotor and feeding behavior, and sleep. More specifically, they are involved in several cellular functions, including proliferation and migration of neural stem cells, vascular reactivity, apoptosis and cytokine secretion. These functions have not been well characterized and the effect of the extracellular microenvironment on their function is also poorly understood.
In molecular biology, extracellular signal-regulated kinases (ERKs) or classical MAP kinases are widely expressed protein kinase intracellular signalling molecules that are involved in functions including the regulation of meiosis, mitosis, and postmitotic functions in differentiated cells. Many different stimuli, including growth factors, cytokines, virus infection, ligands for heterotrimeric G protein-coupled receptors, transforming agents, and carcinogens, activate the ERK pathway.

P2Y receptors are a family of purinergic G protein-coupled receptors, stimulated by nucleotides such as adenosine triphosphate, adenosine diphosphate, uridine triphosphate, uridine diphosphate and UDP-glucose.To date, 8 P2Y receptors have been cloned in humans: P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 and P2Y14.

Wall-associated kinases (WAKs) are one of many classes of plant proteins known to serve as a medium between the extracellular matrix (ECM) and cytoplasm of cell walls. They are serine-threonine kinases that contain epidermal growth factor (EGF) repeats, a cytoplasmic kinase and are located in the cell walls. They provide a linkage between the inner and outer surroundings of cell walls. WAKs are under a group of receptor-like kinases (RLK) that are actively involved in sensory and signal transduction pathways especially in response to foreign attacks by pathogens and in cell development. On the other hand, pectins are an abundant group of complex carbohydrates present in the primary cell wall that play roles in cell growth and development, protection, plant structure and water holding capacity.

Mitogen-activated protein kinase kinase kinase 7 (MAP3K7), also known as TAK1, is an enzyme that in humans is encoded by the MAP3K7 gene.

P2X purinoceptor 7 is a protein that in humans is encoded by the P2RX7 gene.

Mitogen-activated protein kinase 7 also known as MAP kinase 7 is an enzyme that in humans is encoded by the MAPK7 gene.

Dual specificity mitogen-activated protein kinase kinase 6 also known as MAP kinase kinase 6 or MAPK/ERK kinase 6 is an enzyme that in humans is encoded by the MAP2K6 gene, on chromosome 17.

Inositol (1,4,5) trisphosphate 3-kinase (EC 2.7.1.127), abbreviated here as ITP3K, is an enzyme that facilitates a phospho-group transfer from adenosine triphosphate to 1D-myo-inositol 1,4,5-trisphosphate. This enzyme belongs to the family of transferases, specifically those transferring phosphorus-containing groups (phosphotransferases) with an alcohol group as acceptor. The systematic name of this enzyme class is ATP:1D-myo-inositol-1,4,5-trisphosphate 3-phosphotransferase. ITP3K catalyzes the transfer of the gamma-phosphate from ATP to the 3-position of inositol 1,4,5-trisphosphate to form inositol 1,3,4,5-tetrakisphosphate. ITP3K is highly specific for the 1,4,5-isomer of IP3, and it exclusively phosphorylates the 3-OH position, producing Ins(1,3,4,5)P4, also known as inositol tetrakisphosphate or IP4.

C-type lectin domain family 7 member A or Dectin-1 is a protein that in humans is encoded by the CLEC7A gene. CLEC7A is a member of the C-type lectin/C-type lectin-like domain (CTL/CTLD) superfamily. The encoded glycoprotein is a small type II membrane receptor with an extracellular C-type lectin-like domain fold and a cytoplasmic domain with a partial immunoreceptor tyrosine-based activation motif. It functions as a pattern-recognition receptor for a variety of β-1,3-linked and β-1,6-linked glucans from fungi and plants, and in this way plays a role in innate immune response. Expression is found on myeloid dendritic cells, monocytes, macrophages and B cells. Alternate transcriptional splice variants, encoding different isoforms, have been characterized. This gene is closely linked to other CTL/CTLD superfamily members on chromosome 12p13 in the natural killer gene complex region.
P2 receptor may refer to:

P2X purinoceptor 4 is a protein that in humans is encoded by the P2RX4 gene. P2X purinoceptor 4 is a member of the P2X receptor family. P2X receptors are trimeric protein complexes that can be homomeric or heteromeric. These receptors are ligand-gated cation channels that open in response to ATP binding. Each receptor subtype, determined by the subunit composition, varies in its affinity to ATP and desensitization kinetics.

Mitogen-activated protein kinase 4 is an enzyme that in humans is encoded by the MAPK4 gene.
Resistance genes (R-Genes) are genes in plant genomes that convey plant disease resistance against pathogens by producing R proteins. The main class of R-genes consist of a nucleotide binding domain (NB) and a leucine rich repeat (LRR) domain(s) and are often referred to as (NB-LRR) R-genes or NLRs. Generally, the NB domain binds either ATP/ADP or GTP/GDP. The LRR domain is often involved in protein-protein interactions as well as ligand binding. NB-LRR R-genes can be further subdivided into toll interleukin 1 receptor (TIR-NB-LRR) and coiled-coil (CC-NB-LRR).
BRI1-associated receptor kinase 1 is an important plant protein that has diverse functions in plant development.

Purinergic signalling is a form of extracellular signalling mediated by purine nucleotides and nucleosides such as adenosine and ATP. It involves the activation of purinergic receptors in the cell and/or in nearby cells, thereby regulating cellular functions.