
A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a synapse. The cell receiving the signal, or target cell, may be another neuron, but could also be a gland or muscle cell.
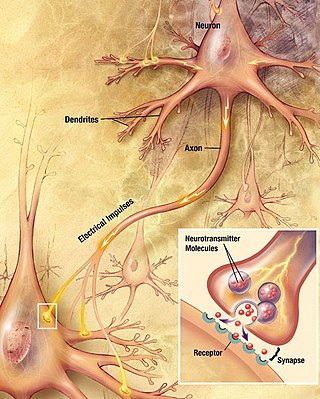
Chemical synapses are biological junctions through which neurons' signals can be sent to each other and to non-neuronal cells such as those in muscles or glands. Chemical synapses allow neurons to form circuits within the central nervous system. They are crucial to the biological computations that underlie perception and thought. They allow the nervous system to connect to and control other systems of the body.

A dendritic spine is a small membranous protrusion from a neuron's dendrite that typically receives input from a single axon at the synapse. Dendritic spines serve as a storage site for synaptic strength and help transmit electrical signals to the neuron's cell body. Most spines have a bulbous head, and a thin neck that connects the head of the spine to the shaft of the dendrite. The dendrites of a single neuron can contain hundreds to thousands of spines. In addition to spines providing an anatomical substrate for memory storage and synaptic transmission, they may also serve to increase the number of possible contacts between neurons. It has also been suggested that changes in the activity of neurons have a positive effect on spine morphology.

In neuroscience, long-term potentiation (LTP) is a persistent strengthening of synapses based on recent patterns of activity. These are patterns of synaptic activity that produce a long-lasting increase in signal transmission between two neurons. The opposite of LTP is long-term depression, which produces a long-lasting decrease in synaptic strength.
In neuroscience, synaptic plasticity is the ability of synapses to strengthen or weaken over time, in response to increases or decreases in their activity. Since memories are postulated to be represented by vastly interconnected neural circuits in the brain, synaptic plasticity is one of the important neurochemical foundations of learning and memory.
An inhibitory postsynaptic potential (IPSP) is a kind of synaptic potential that makes a postsynaptic neuron less likely to generate an action potential. The opposite of an inhibitory postsynaptic potential is an excitatory postsynaptic potential (EPSP), which is a synaptic potential that makes a postsynaptic neuron more likely to generate an action potential. IPSPs can take place at all chemical synapses, which use the secretion of neurotransmitters to create cell-to-cell signalling. EPSPs and IPSPs compete with each other at numerous synapses of a neuron. This determines whether an action potential occurring at the presynaptic terminal produces an action potential at the postsynaptic membrane. Some common neurotransmitters involved in IPSPs are GABA and glycine.

An excitatory synapse is a synapse in which an action potential in a presynaptic neuron increases the probability of an action potential occurring in a postsynaptic cell. Neurons form networks through which nerve impulses travels, each neuron often making numerous connections with other cells of neurons. These electrical signals may be excitatory or inhibitory, and, if the total of excitatory influences exceeds that of the inhibitory influences, the neuron will generate a new action potential at its axon hillock, thus transmitting the information to yet another cell.

Brain-derived neurotrophic factor (BDNF), or abrineurin, is a protein that, in humans, is encoded by the BDNF gene. BDNF is a member of the neurotrophin family of growth factors, which are related to the canonical nerve growth factor (NGF), a family which also includes NT-3 and NT-4/NT-5. Neurotrophic factors are found in the brain and the periphery. BDNF was first isolated from a pig brain in 1982 by Yves-Alain Barde and Hans Thoenen.

Podocytes are cells in Bowman's capsule in the kidneys that wrap around capillaries of the glomerulus. Podocytes make up the epithelial lining of Bowman's capsule, the third layer through which filtration of blood takes place. Bowman's capsule filters the blood, retaining large molecules such as proteins while smaller molecules such as water, salts, and sugars are filtered as the first step in the formation of urine. Although various viscera have epithelial layers, the name visceral epithelial cells usually refers specifically to podocytes, which are specialized epithelial cells that reside in the visceral layer of the capsule.
Synaptogenesis is the formation of synapses between neurons in the nervous system. Although it occurs throughout a healthy person's lifespan, an explosion of synapse formation occurs during early brain development, known as exuberant synaptogenesis. Synaptogenesis is particularly important during an individual's critical period, during which there is a certain degree of synaptic pruning due to competition for neural growth factors by neurons and synapses. Processes that are not used, or inhibited during their critical period will fail to develop normally later on in life.

The postsynaptic density (PSD) is a protein dense specialization attached to the postsynaptic membrane. PSDs were originally identified by electron microscopy as an electron-dense region at the membrane of a postsynaptic neuron. The PSD is in close apposition to the presynaptic active zone and ensures that receptors are in close proximity to presynaptic neurotransmitter release sites. PSDs vary in size and composition among brain regions, and have been studied in great detail at glutamatergic synapses. Hundreds of proteins have been identified in the postsynaptic density, including glutamate receptors, scaffold proteins, and many signaling molecules.
Metaplasticity is a term originally coined by W.C. Abraham and M.F. Bear to refer to the plasticity of synaptic plasticity. Until that time synaptic plasticity had referred to the plastic nature of individual synapses. However this new form referred to the plasticity of the plasticity itself, thus the term meta-plasticity. The idea is that the synapse's previous history of activity determines its current plasticity. This may play a role in some of the underlying mechanisms thought to be important in memory and learning such as long-term potentiation (LTP), long-term depression (LTD) and so forth. These mechanisms depend on current synaptic "state", as set by ongoing extrinsic influences such as the level of synaptic inhibition, the activity of modulatory afferents such as catecholamines, and the pool of hormones affecting the synapses under study. Recently, it has become clear that the prior history of synaptic activity is an additional variable that influences the synaptic state, and thereby the degree, of LTP or LTD produced by a given experimental protocol. In a sense, then, synaptic plasticity is governed by an activity-dependent plasticity of the synaptic state; such plasticity of synaptic plasticity has been termed metaplasticity. There is little known about metaplasticity, and there is much research currently underway on the subject, despite its difficulty of study, because of its theoretical importance in brain and cognitive science. Most research of this type is done via cultured hippocampus cells or hippocampal slices.

In the nervous system, a synapse is a structure that permits a neuron to pass an electrical or chemical signal to another neuron or to the target effector cell.

Neuroligin (NLGN), a type I membrane protein, is a cell adhesion protein on the postsynaptic membrane that mediates the formation and maintenance of synapses between neurons. Neuroligins act as ligands for β-neurexins, which are cell adhesion proteins located presynaptically. Neuroligin and β-neurexin "shake hands", resulting in the connection between two neurons and the production of a synapse. Neuroligins also affect the properties of neural networks by specifying synaptic functions, and they mediate signalling by recruiting and stabilizing key synaptic components. Neuroligins interact with other postsynaptic proteins to localize neurotransmitter receptors and channels in the postsynaptic density as the cell matures. Additionally, neuroligins are expressed in human peripheral tissues and have been found to play a role in angiogenesis. In humans, alterations in genes encoding neuroligins are implicated in autism and other cognitive disorders. Antibodies in a mother from previous male pregnancies against neuroligin 4 from the Y chromosome increase the probability of homosexuality in male offspring.

Nonsynaptic plasticity is a form of neuroplasticity that involves modification of ion channel function in the axon, dendrites, and cell body that results in specific changes in the integration of excitatory postsynaptic potentials and inhibitory postsynaptic potentials. Nonsynaptic plasticity is a modification of the intrinsic excitability of the neuron. It interacts with synaptic plasticity, but it is considered a separate entity from synaptic plasticity. Intrinsic modification of the electrical properties of neurons plays a role in many aspects of plasticity from homeostatic plasticity to learning and memory itself. Nonsynaptic plasticity affects synaptic integration, subthreshold propagation, spike generation, and other fundamental mechanisms of neurons at the cellular level. These individual neuronal alterations can result in changes in higher brain function, especially learning and memory. However, as an emerging field in neuroscience, much of the knowledge about nonsynaptic plasticity is uncertain and still requires further investigation to better define its role in brain function and behavior.

Activity-regulated cytoskeleton-associated protein is a plasticity protein that in humans is encoded by the ARC gene. The gene is believed to derive from a retrotransposon. The protein is found in the neurons of tetrapods and other animals where it can form virus-like capsids that transport RNA between neurons.
Synaptic tagging, or the synaptic tagging hypothesis, has been proposed to explain how neural signaling at a particular synapse creates a target for subsequent plasticity-related product (PRP) trafficking essential for sustained LTP and LTD. Although the molecular identity of the tags remains unknown, it has been established that they form as a result of high or low frequency stimulation, interact with incoming PRPs, and have a limited lifespan.
Actin remodeling is a biochemical process in cells. In the actin remodeling of neurons, the protein actin is part of the process to change the shape and structure of dendritic spines. G-actin is the monomer form of actin, and is uniformly distributed throughout the axon and the dendrite. F-actin is the polymer form of actin, and its presence in dendritic spines is associated with their change in shape and structure. Actin plays a role in the formation of new spines as well as stabilizing spine volume increase. The changes that actin brings about lead to the formation of new synapses as well as increased cell communication.

Synaptic stabilization is crucial in the developing and adult nervous systems and is considered a result of the late phase of long-term potentiation (LTP). The mechanism involves strengthening and maintaining active synapses through increased expression of cytoskeletal and extracellular matrix elements and postsynaptic scaffold proteins, while pruning less active ones. For example, cell adhesion molecules (CAMs) play a large role in synaptic maintenance and stabilization. Gerald Edelman discovered CAMs and studied their function during development, which showed CAMs are required for cell migration and the formation of the entire nervous system. In the adult nervous system, CAMs play an integral role in synaptic plasticity relating to learning and memory.
An axo-axonic synapse is a type of synapse, formed by one neuron projecting its axon terminals onto another neuron's axon.
















