Related Research Articles
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance's molecules, adsorbing substances can become physically changed, e.g. changing in volume, boiling point, viscosity or some other physical characteristic or property of the substance. For example, a finely dispersed hygroscopic powder, such as a salt, may become clumpy over time due to collection of moisture from the surrounding environment.

Drosophila melanogaster is a species of fly in the family Drosophilidae. The species is often referred to as the fruit fly or lesser fruit fly, or less commonly the "vinegar fly", "pomace fly", or "banana fly". In the wild, D. melanogaster are attracted to rotting fruit and fermenting beverages, and are often found in orchards, kitchens and pubs.
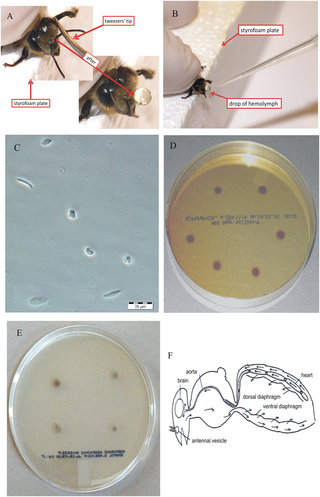
Hemolymph, or haemolymph, is a fluid, analogous to the blood in vertebrates, that circulates in the interior of the arthropod (invertebrate) body, remaining in direct contact with the animal's tissues. It is composed of a fluid plasma in which hemolymph cells called hemocytes are suspended. In addition to hemocytes, the plasma also contains many chemicals. It is the major tissue type of the open circulatory system characteristic of arthropods. In addition, some non-arthropods such as mollusks possess a hemolymphatic circulatory system.

Desiccation is the state of extreme dryness, or the process of extreme drying. A desiccant is a hygroscopic substance that induces or sustains such a state in its local vicinity in a moderately sealed container. The word desiccation comes from Latin de- 'thoroughly', and siccare 'to dry'.

In animal dormancy, diapause is the delay in development in response to regular and recurring periods of adverse environmental conditions. It is a physiological state with very specific initiating and inhibiting conditions. The mechanism is a means of surviving predictable, unfavorable environmental conditions, such as temperature extremes, drought, or reduced food availability. Diapause is observed in all the life stages of arthropods, especially insects.
Cold hardening is the physiological and biochemical process by which an organism prepares for cold weather.

Cryptobiosis or anabiosis is a metabolic state in extremophilic organisms in response to adverse environmental conditions such as desiccation, freezing, and oxygen deficiency. In the cryptobiotic state, all measurable metabolic processes stop, preventing reproduction, development, and repair. When environmental conditions return to being hospitable, the organism will return to its metabolic state of life as it was prior to cryptobiosis.

Desert ecology is the study of interactions between both biotic and abiotic components of desert environments. A desert ecosystem is defined by interactions between organisms, the climate in which they live, and any other non-living influences on the habitat. Deserts are arid regions that are generally associated with warm temperatures; however, cold deserts also exist. Deserts can be found in every continent, with the largest deserts located in Antarctica, the Arctic, Northern Africa, and the Middle East.
Enantiostasis is the ability of an open system, especially a living organism, to maintain and conserve its metabolic and physiological functions in response to variations in an unstable environment. Estuarine organisms typically undergo enantiostasis in order to survive with constantly changing salt concentrations. The Australian NSW Board of Studies defines the term in its Biology syllabus as "the maintenance of metabolic and physiological functions in response to variations in the environment".

Selaginella lepidophylla, also known as a resurrection plant, is a species of desert plant in the spikemoss family (Selaginellaceae). It is native to the Chihuahuan Desert of the United States and Mexico. S. lepidophylla is renowned for its ability to survive almost complete desiccation. Resurrection plants are vascular rooted plants capable of surviving extreme desiccation, then resuming normal metabolic activity upon rehydration. The plant's hydro-responsive movements are governed by stem moisture content, tissue properties and a graded distribution of lignified cells affecting concentric stem stiffness and spiraling. During dry weather in its native habitat, its stems curl into a tight ball, uncurling only when exposed to moisture.

Belgica antarctica, the Antarctic midge, is a species of flightless midge, endemic to the continent of Antarctica. At 2–6 mm (0.08–0.2 in) long, it is the largest purely terrestrial animal native to the continent. It also has the smallest known insect genome as of 2014, with only 99 million base pairs of nucleotides and about 13500 genes. It is the only insect that can survive year-round in Antarctica.

Insect winter ecology describes the overwinter survival strategies of insects, which are in many respects more similar to those of plants than to many other animals, such as mammals and birds. Unlike those animals, which can generate their own heat internally (endothermic), insects must rely on external sources to provide their heat (ectothermic). Thus, insects persisting in winter weather must tolerate freezing or rely on other mechanisms to avoid freezing. Loss of enzymatic function and eventual freezing due to low temperatures daily threatens the livelihood of these organisms during winter. Not surprisingly, insects have evolved a number of strategies to deal with the rigors of winter temperatures in places where they would otherwise not survive.

Phyllomedusa sauvagii, the waxy monkey leaf frog or waxy monkey tree frog, is a species of frog in the subfamily Phyllomedusinae. It is native to South America, where it occurs in Argentina, Bolivia, Paraguay and Brazil. This species is arboreal, living in the vegetation of the Gran Chaco.
A xerophyte is a species of plant that has adaptations to survive in an environment with little liquid water. Examples of xerophytes include cacti, pineapple and some gymnosperm plants. The morphology and physiology of xerophytes are adapted to conserve water during dry periods. Some species called resurrection plants can survive long periods of extreme dryness or desiccation of their tissues, during which their metabolic activity may effectively shut down. Plants with such morphological and physiological adaptations are said to be xeromorphic. Xerophytes such as cacti are capable of withstanding extended periods of dry conditions as they have deep-spreading roots and capacity to store water. Their waxy, thorny leaves prevent loss of moisture.
Discontinuous gas-exchange cycles (DGC), also called discontinuous ventilation or discontinuous ventilatory cycles, follow one of several patterns of arthropod gas exchange that have been documented primarily in insects; they occur when the insect is at rest. During DGC, oxygen (O2) uptake and carbon dioxide (CO2) release from the whole insect follow a cyclical pattern characterized by periods of little to no release of CO2 to the external environment. Discontinuous gas exchange is traditionally defined in three phases, whose names reflect the behaviour of the spiracles: the closed phase, the flutter phase, and the open phase.
Polypedilum vanderplanki or the sleeping chironomid, is a dipteran in the family Chironomidae. It occurs in the semi-arid regions of the African continent. Its larvae are found in small tubular nests in the mud at the bottom of temporary pools that frequently dry out during the lifetime of P. vanderplanki larvae. Under these conditions, the larvae's body desiccates to as low as 3% water content by weight. In the dehydrated state the larvae become impervious to many extreme environmental conditions, and can survive temperatures from 3 K to up to 375 K, very high levels of gamma-rays, and exposure to vacuum. It is one of few metazoans that can withstand near complete desiccation (anhydrobiosis) in order to survive adverse environmental conditions. Slow desiccation enabled larvae to synthesize 38 μg trehalose/individual, and all of them recovered after rehydration, whereas larvae that were dehydrated 3 times faster accumulated only 6.8 μg trehalose/individual and none of them revived after rehydration. Late Embryo Abundant (LEA), anti-oxidant, and heat-shock proteins may also be involved in survival. This species is considered the most cold-tolerant insect species, able to survive liquid helium (−270 °C) exposure for up to 5 min. with a 100% survival rate when desiccated to 8% water content.

Hemideina maori, also known as the mountain stone wētā, is a wētā of the family Anostostomatidae. They are a large, flightless, nocturnal orthopteran endemic to New Zealand. Mountain stone wētā are long lived and are found on many central mountain ranges in New Zealand's South Island.
Abdominal pigmentation in Drosophila melanogaster is a morphologically simple but highly variable trait that often has adaptive significance. Pigmentation has extensively been studied in Drosophila melanogaster. It has been used as a model for understanding the development and evolution of morphological phenotypes.
CYP303A1 is an insect gene belongs to the cytochrome P450 family, first found in Drosophila melanogaster, highly expressed in pupal stage. Its ortholog also found in Locusta migratoria.
Tardigrade specific proteins are types of intrinsically disordered proteins specific to tardigrades. These proteins help tardigrades survive desiccation, one of the adaptations which contribute to tardigrade's extremotolerant nature. Tardigrade specific proteins are strongly influenced by their environment, leading to adaptive malleability across a variety of extreme abiotic environments.
References
- 1 2 3 Chown, S.L.; Nicolson, S.W. (2004). "Insect Physiological Ecology". New York: Oxford University Press.
- ↑ Yuki Nakamura and Yonghua Li-Beisson (Editors) ISBN 9783319259772, Springer , p. 185, at Google Books
- 1 2 3 4 Gray, E.M.; Bradley, T.J. (2005). "Physiology of desiccation resistance in Anopheles gambiae and Anopheles arabiensis". American Journal of Tropical Medicine and Hygiene. 73 (3): 553–559. doi: 10.4269/ajtmh.2005.73.553 . PMID 16172480.
- ↑ Graves, J. L.; Toolson, E. C.; Jeong, C.; Vu, L.N. & Rose, M.R. (1992). "Desiccation, flight, glycogen and postponed senescence in Drosophila melanogaster". Physiological Zoology. 65 (2): 268–286. doi:10.1086/physzool.65.2.30158253. S2CID 29889650.
- ↑ Gefen, E.; Marlon, A.J.; Gibbs, A.G. (2006). "Selection for desiccation resistance in adult Drosophila melanogaster affects larval development and metabolite accumulation". Journal of Experimental Biology. 209 (Pt 17): 3293–3300. doi: 10.1242/jeb.02397 . PMID 16916965.
- 1 2 3 Folk, D.G.; Han, C.; Bradley, T.J. (2001). "Water acquisition and partitioning in Drosophila melanogaster: effects of selection for desiccation-resistance". Journal of Experimental Biology. 204 (Pt 19): 3323–3331. doi:10.1242/jeb.204.19.3323. PMID 11606606.
- ↑ Mellanby, K. (1934). "The evaporation of water from insects". Journal of Experimental Biology. 10 (3): 317–333. doi:10.1111/j.1469-185x.1935.tb00487.x. S2CID 84993444.
- ↑ Machin J. 1968. The Permeability of the Epiphragm of Terrestrial Snails to Water Vapor. Biological Bulletin, Vol. 134, No. 1 (Feb., 1968), pp. 87-95
- ↑ Wigglesworth, V.B. (1984). "Insect Physiology". Chapman and Hall, NY.
- 1 2 3 Gibbs, A.G. (2002). "Lipid melting and cuticular permeability: new insights into an old problem". Journal of Insect Physiology. 48 (4): 391–400. Bibcode:2002JInsP..48..391G. doi:10.1016/S0022-1910(02)00059-8. PMID 12770088.
- 1 2 Treherne, J.E.; Willmer, P.G. (1975). "Hormonal control of integumentary water loss: evidence for a novel neuroendocrine system in an insect (Periplaneta americana)". Journal of Experimental Biology. 63 (1): 143–159. doi:10.1242/jeb.63.1.143. PMID 1159358.
- 1 2 Snyder, G.K.; Sheafor, B.; Scholnick, D.; Farrelly, C. (1995). "Gas Exchange in the Insect Tracheal System". Journal of Theoretical Biology. 172 (3): 199–207. Bibcode:1995JThBi.172..199S. doi:10.1006/jtbi.1995.0016. PMID 7715192.
- 1 2 Bursell, E. (1960). "Loss of water by excretion and defaecation in the Tsetse fly". Journal of Experimental Biology. 37 (4): 689–697. doi:10.1242/jeb.37.4.689.
- ↑ Willmer, P.; Stone, G.; Johnston, I.A. (2000). "Environmental physiology of animals". New York: Blackwell Publishing.
- 1 2 Renault, D.; Coray, Y. (2004). "Water loss of male and female Alphitobius diaperinus (Coleoptera: Tenebrionidae) maintained under dry conditions". European Journal of Entomology. 101 (3): 491–494. doi: 10.14411/eje.2004.069 .
- ↑ Prince, G.J.; Parsons, P.A. (1977). "Adaptive Behaviour of Drosophila Adults in Relation to Temperature and Humidity". Australian Journal of Zoology. 25 (2): 285–290. doi:10.1071/ZO9770285.
- ↑ Halffter, G.; Edmonds, W.D. (1982). The Nesting Behavior of Dung Beetles (Scarabaeinae): An Ecological and Evolutive Approach. Mexico City, Mexico: Instituto de Ecologia.
- ↑ Rowley, M.; Hanson, F. (2007). "Humidity detection and hygropreference behavior in larvae of the tobacco hornworm, Manduca sexta". Journal of Insect Science. 7 (39): 1–10. doi:10.1673/031.007.3901. PMC 2999434 . PMID 20302460.
- 1 2 Watanbe, M.; Kikawada, T.; Okuda, T. (2003). "Increase of internal ion concentration triggers trehalose synthesis associated with cryptobiosis in larvae of Polypedilum vanderplanki". Journal of Experimental Biology. 206 (Pt 13): 2281–2286. doi: 10.1242/jeb.00418 . PMID 12771176.