
Macrolides are a class of mostly natural products with a large macrocyclic lactone ring to which one or more deoxy sugars, usually cladinose and desosamine, may be attached. The lactone rings are usually 14-, 15-, or 16-membered. Macrolides belong to the polyketide class of natural products. Some macrolides have antibiotic or antifungal activity and are used as pharmaceutical drugs. Rapamycin is also a macrolide and was originally developed as an antifungal, but has since been used as an immunosuppressant drug and is being investigated as a potential longevity therapeutic.

Teicoplanin is an semisynthetic glycopeptide antibiotic with a spectrum of activity similar to vancomycin. Its mechanism of action is to inhibit bacterial cell wall peptidoglycan synthesis. It is used in the prophylaxis and treatment of serious infections caused by Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus and Enterococcus faecalis.
In organic chemistry, polyketides are a class of natural products derived from a precursor molecule consisting of a chain of alternating ketone and methylene groups: [−C(=O)−CH2−]n. First studied in the early 20th century, discovery, biosynthesis, and application of polyketides has evolved. It is a large and diverse group of secondary metabolites caused by its complex biosynthesis which resembles that of fatty acid synthesis. Because of this diversity, polyketides can have various medicinal, agricultural, and industrial applications. Many polyketides are medicinal or exhibit acute toxicity. Biotechnology has enabled discovery of more naturally-occurring polyketides and evolution of new polyketides with novel or improved bioactivity.

Novobiocin, also known as albamycin, is an aminocoumarin antibiotic that is produced by the actinomycete Streptomyces niveus, which has recently been identified as a subjective synonym for S. spheroides a member of the class Actinomycetia. Other aminocoumarin antibiotics include clorobiocin and coumermycin A1. Novobiocin was first reported in the mid-1950s.

Lincosamides are a class of antibiotics, which include lincomycin, clindamycin, and pirlimycin.

Oleandomycin is a macrolide antibiotic. It is synthesized from strains of Streptomyces antibioticus. It is weaker than erythromycin.

Doxorubicin (DXR) is a 14-hydroxylated version of daunorubicin, the immediate precursor of DXR in its biosynthetic pathway. Daunorubicin is more abundantly found as a natural product because it is produced by a number of different wild type strains of Streptomyces. In contrast, only one known non-wild type species, Streptomyces peucetius subspecies caesius ATCC 27952, was initially found to be capable of producing the more widely used doxorubicin. This strain was created by Arcamone et al. in 1969 by mutating a strain producing daunorubicin, but not DXR, at least in detectable quantities. Subsequently, Hutchinson's group showed that under special environmental conditions, or by the introduction of genetic modifications, other strains of streptomyces can produce doxorubicin. His group has also cloned many of the genes required for DXR production, although not all of them have been fully characterized. In 1996, Strohl's group discovered, isolated and characterized dox A, the gene encoding the enzyme that converts daunorubicin into DXR. By 1999, they produced recombinant Dox A, a Cytochrome P450 oxidase, and found that it catalyzes multiple steps in DXR biosynthesis, including steps leading to daunorubicin. This was significant because it became clear that all daunorubicin producing strains have the necessary genes to produce DXR, the much more therapeutically important of the two. Hutchinson's group went on to develop methods to improve the yield of DXR, from the fermentation process used in its commercial production, not only by introducing Dox A encoding plasmids, but also by introducing mutations to deactivate enzymes that shunt DXR precursors to less useful products, for example baumycin-like glycosides. Some triple mutants, that also over-expressed Dox A, were able to double the yield of DXR. This is of more than academic interest because at that time DXR cost about $1.37 million per kg and current production in 1999 was 225 kg per annum. More efficient production techniques have brought the price down to $1.1 million per kg for the non-liposomal formulation. Although DXR can be produced semi-synthetically from daunorubicin, the process involves electrophilic bromination and multiple steps and the yield is poor. Since daunorubicin is produced by fermentation, it would be ideal if the bacteria could complete DXR synthesis more effectively.
The enzyme dTDP-glucose 4,6-dehydratase (EC 4.2.1.46) catalyzes the chemical reaction
In enzymology, an erythronolide synthase is an enzyme that catalyzes the chemical reaction
The aminocyclitols are compounds related to cyclitols. They possess features of relative and absolute configuration that are characteristic of their class and have been extensively studied; but these features are not clearly displayed by general methods of stereochemical nomenclature, so that special methods of specifying their configuration are justified and have long been used. In other than stereochemical respects, their nomenclature should follow the general rules of organic chemistry.

Pristinamycin IIA is a macrolide antibiotic. It is a member of the streptogramin A group of antibiotics and one component of pristinamycin. Pristinamycin IIA was first isolated from the Streptomyces virginiae, but has been isolated from other microorganisms and thus has been given a variety of other names such as Virginiamycin M1, Mikamycin A, and Streptogramin A. Pristinamycin IIA structure was determined by chemical and instrumental techniques, including X-ray crystallography. Pristinamycin IIA is of interest from a biosynthetic viewpoint because it contains the unusual dehydroproline and oxazole ring systems. The only experimental evidence bearing on the formation of the oxazole ring is found in work on the biosynthesis of the alkaloid annuloline.
6-deoxyerythronolide B hydroxylase is an Actinomycetota Cytochrome P450 enzyme originally from Saccharopolyspora erythraea, catalyzes the 6S-hydroxylate of 6-deoxyerythronolide B (6-DEB) to erythronolide B (EB) which is the first step of biosynthesis of the macrolide antibiotic erythromycin. This bacterial enzyme belongs to CYP family CYP107, with the CYP Symbol CYP107A1.
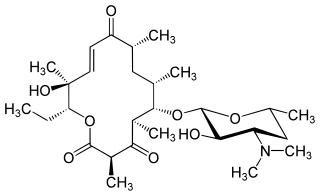
Pikromycin was studied by Brokmann and Hekel in 1951 and was the first antibiotic macrolide to be isolated. Pikromycin is synthesized through a type I polyketide synthase system in Streptomyces venezuelae, a species of Gram-positive bacterium in the genus Streptomyces. Pikromycin is derived from narbonolide, a 14-membered ring macrolide. Along with the narbonolide backbone, pikromycin includes a desosamine sugar and a hydroxyl group. Although Pikromycin is not a clinically useful antibiotic, it can be used as a raw material to synthesize antibiotic ketolide compounds such as ertythromycins and new epothilones.
DTDP-3-amino-3,4,6-trideoxy-alpha-D-glucopyranose N,N-dimethyltransferase is an enzyme with systematic name S-adenosyl-L-methionine:dTDP-3-amino-3,4,6-trideoxy-alpha-D-glucopyranose 3-N,N-dimethyltransferase. This enzyme catalyses the following chemical reaction
DTDP-3-amino-3,6-dideoxy-alpha-D-glucopyranose N,N-dimethyltransferase is an enzyme with systematic name S-adenosyl-L-methionine:dTDP-3-amino-3,6-dideoxy-alpha-D-glucopyranose 3-N,N-dimethyltransferase. This enzyme catalyses the following chemical reaction
Glycosyltransferase DesVII is an enzyme with systematic name dTDP-3-dimethylamino-3,4,6-trideoxy-alpha-D-glucopyranose:10-deoxymethynolide 3-dimethylamino-4,6-dideoxy-alpha-D-glucosyltransferase. This enzyme catalyses the following chemical reaction
Desosaminyl transferase EryCIII is an enzyme with systematic name dTDP-3-dimethylamino-4,6-dideoxy-alpha-D-glucopyranose:3-alpha-mycarosylerythronolide B 3-dimethylamino-4,6-dideoxy-alpha-D-glucosyltransferase. This enzyme catalyses the following chemical reaction

Carbomycin, also known as magnamycin, is a colorless, optically active crystalline macrolide antibiotic with the molecular formula C42H67N O16. It is derived from the bacterium Streptomyces halstedii and active in inhibiting the growth of Gram-positive bacteria and "certain Mycoplasma strains." Its structure was first proposed by Robert Woodward in 1957 and was subsequently corrected in 1965.

C-1027 or lidamycin is an antitumor antibiotic consisting of a complex of an enediyne chromophore and an apoprotein. It shows antibiotic activity against most Gram-positive bacteria. It is one of the most potent cytotoxic molecules known, due to its induction of a higher ratio of DNA double-strand breaks than single-strand breaks.

Enterocin and its derivatives are bacteriocins synthesized by the lactic acid bacteria, Enterococcus. This class of polyketide antibiotics are effective against foodborne pathogens including L. monocytogenes, Listeria, and Bacillus. Due to its proteolytic degradability in the gastrointestinal tract, enterocin is used for controlling foodborne pathogens via human consumption.











