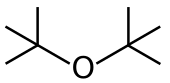
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name 2-Methyl-2-[(2-methylpropan-2-yl)oxy]propane | |
| Other names 2-tert-Butoxy-2-methylpropane Di-tert-butyl ether | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.197.715 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C8H18O | |
| Molar mass | 130.231 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.7658 g/cm3 [2] |
| Melting point | −61 °C (−78 °F; 212 K) [3] |
| Boiling point | 107.2 °C (225.0 °F; 380.3 K) [2] |
| Vapor pressure | 3730 Pa (at 22 °C) [4] |
| Thermochemistry | |
Heat capacity (C) | 276.1 J·mol−1·K−1 |
Std enthalpy of formation (ΔfH⦵298) | −399.6 kJ·mol−1 |
| Hazards | |
| Flash point | −3 °C (27 °F; 270 K) |
| 365 °C (689 °F; 638 K) | |
| Explosive limits | >0.4% |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Di-tert-butyl ether is a tertiary ether, primarily of theoretical interest as the simplest member of the class of di-tertiary ethers. Di-tertiary ethers are notoriously challenging to prepare because conventional SN,2 and dehydration methods favor elimination; as late as 1941, the existence of the molecule remained in doubt. [5]
A mediocre-yielding synthesis for di-tert-butyl ether alkylates silver carbonate with tert-butyl chloride. [5]