
In organic chemistry, an imine is a functional group or organic compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.

Amidines are organic compounds with the functional group RC(NR)NR2, where the R groups can be the same or different. They are the imine derivatives of amides (RC(O)NR2). The simplest amidine is formamidine, HC(=NH)NH2.
Organopalladium chemistry is a branch of organometallic chemistry that deals with organic palladium compounds and their reactions. Palladium is often used as a catalyst in the reduction of alkenes and alkynes with hydrogen. This process involves the formation of a palladium-carbon covalent bond. Palladium is also prominent in carbon-carbon coupling reactions, as demonstrated in tandem reactions.

1,3,5,7-Cyclooctatetraene (COT) is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as [8]annulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature. Because of its stoichiometric relationship to benzene, COT has been the subject of much research and some controversy.
Cycloheptatriene (CHT) is an organic compound with the formula C7H8. It is a closed ring of seven carbon atoms joined by three double bonds (as the name implies) and four single bonds. This colourless liquid has been of recurring theoretical interest in organic chemistry. It is a ligand in organometallic chemistry and a building block in organic synthesis. Cycloheptatriene is not aromatic, as reflected by the nonplanarity of the methylene bridge (-CH2-) with respect to the other atoms; however the related tropylium cation is.

tert-Butyllithium is a chemical compound with the formula (CH3)3CLi. As an organolithium compound, it has applications in organic synthesis since it is a strong base, capable of deprotonating many carbon molecules, including benzene. tert-Butyllithium is available commercially as solutions in hydrocarbons (such as pentane); it is not usually prepared in the laboratory.
1,5-Cyclooctadiene is a cyclic hydrocarbon with the chemical formula C8H12, specifically [−(CH2)2−CH=CH−]2.

Tebbe's reagent is the organometallic compound with the formula (C5H5)2TiCH2ClAl(CH3)2. It is used in the methylidenation of carbonyl compounds, that is it converts organic compounds containing the R2C=O group into the related R2C=CH2 derivative. It is a red solid that is pyrophoric in the air, and thus is typically handled with air-free techniques. It was originally synthesized by Fred Tebbe at DuPont Central Research.

Diphenylacetylene is the chemical compound C6H5C≡CC6H5. The molecule consists of two phenyl groups attached to a C2 unit. A colorless solid, it is used as a building block in organic synthesis and as a ligand in organometallic chemistry.
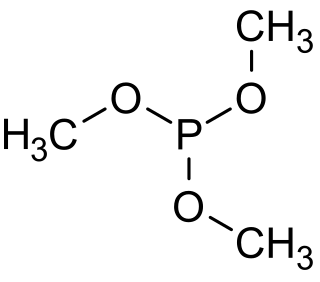
Trimethyl phosphite is an organophosphorus compound with the formula P(OCH3)3, often abbreviated P(OMe)3. It is a colorless liquid with a highly pungent odor. It is the simplest phosphite ester and finds used as a ligand in organometallic chemistry and as a reagent in organic synthesis. The molecule features a pyramidal phosphorus(III) center bound to three methoxy groups.

Bis(cyclooctadiene)nickel(0) is the organonickel compound with the formula Ni(C8H12)2, also written Ni(cod)2. It is a diamagnetic coordination complex featuring tetrahedral nickel(0) bound to the alkene groups in two 1,5-cyclooctadiene ligands. This highly air-sensitive yellow solid is a common source of Ni(0) in chemical synthesis.

1,2-Diphenyl-1,2-ethylenediamine, DPEN, is an organic compound with the formula H2NCHPhCHPhNH2, where Ph is phenyl (C6H5). DPEN exists as three stereoisomers: meso and two enantiomers S,S- and R,R-. The chiral diastereomers are used in asymmetric hydrogenation. Both diastereomers are bidentate ligands.
Organoiron chemistry is the chemistry of iron compounds containing a carbon-to-iron chemical bond. Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. Although iron is generally less active in many catalytic applications, it is less expensive and "greener" than other metals. Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl, but hard ligands such as amines are employed as well.

Phosphinooxazolines are a class of chiral ligands used in asymmetric catalysis. Colorless solids, PHOX ligands feature a tertiary phosphine group, often diphenyl, and an oxazoline ligand in the ortho position. The oxazoline, which carries the stereogenic center, coordinates through nitrogen, the result being that PHOX ligands are P,N-chelating ligands. Most phosphine ligands used in asymmetric catalysis are diphosphines, so the PHOX ligands are distinctive. Some evidence exists that PHOX ligands are hemilabile.
Diimines are organic compounds containing two imine (RCH=NR') groups. Common derivatives are 1,2-diimines and 1,3-diimines. These compounds are used as ligands, but they are also precursors to other organic compounds.

2-Phenylpyridine is an organic compound with the formula C6H5C5H4N (or C11H9N). It is a colourless viscous liquid. The compound and related derivatives have attracted interest as precursors to highly fluorescent metal complexes of possible value as organic light emitting diodes (OLEDs).

Transition-metal allyl complexes are coordination complexes with allyl and its derivatives as ligands. Allyl is the radical with the connectivity CH2CHCH2, although as a ligand it is usually viewed as an allyl anion CH2=CH−CH2−, which is usually described as two equivalent resonance structures.

1,5-Diaza-3,7-diphosphacyclooctanes are organophosphorus compounds with the formula [R'NCH2P(R)CH2]2, often abbreviated PR2NR'2. They are air-sensitive white solids that are soluble in organic solvents. The ligands exist as meso and d,l-diastereomers, but only the meso forms function as bidentate ligands.

Xylylene dibromide is an organic compound with the formula C6H4(CH2Br)2. It is an off-white solid that, like other benzyl halides, is strongly lachrymatory. It is a useful reagent owing to the convenient reactivity of the two C-Br bonds. Two other isomers are known, para- and meta-xylylene dibromide.
Transition metal complexes of 2,2'-bipyridine are coordination complexes containing one or more 2,2'-bipyridine ligands. Complexes have been described for all of the transition metals. Although few have any practical value, these complexes have been influential. 2,2'-Bipyridine (bipy) is classified as a diimine ligand. Unlike the structures of pyridine complexes, the two rings in bipy are coplanar, which facilitates electron delocalization. As a consequence of this delocalization, bipy complexes often exhibit distinctive optical and redox properties.















