In chemistry, halogenation is a chemical reaction which introduces one or more halogens into a chemical compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens. Halides are also commonly introduced using salts of the halides and halogen acids. Many specialized reagents exist for and introducing halogens into diverse substrates, e.g. thionyl chloride.

Hydrogen bromide is the inorganic compound with the formula HBr. It is a hydrogen halide consisting of hydrogen and bromine. A colorless gas, it dissolves in water, forming hydrobromic acid, which is saturated at 68.85% HBr by weight at room temperature. Aqueous solutions that are 47.6% HBr by mass form a constant-boiling azeotrope mixture that boils at 124.3 °C (255.7 °F). Boiling less concentrated solutions releases H2O until the constant-boiling mixture composition is reached.
The Hofmann rearrangement is the organic reaction of a primary amide to a primary amine with one less carbon atom. The reaction involves oxidation of the nitrogen followed by rearrangement of the carbonyl and nitrogen to give an isocyanate intermediate. The reaction can form a wide range of products, including alkyl and aryl amines.
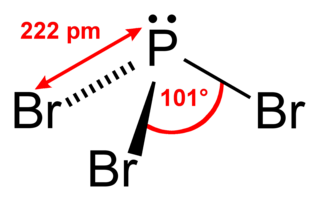
Phosphorus tribromide is a colourless liquid with the formula PBr3. The liquid fumes in moist air due to hydrolysis and has a penetrating odour. It is used in the laboratory for the conversion of alcohols to alkyl bromides.
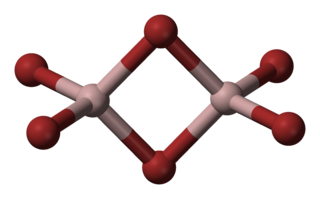
Aluminium bromide is any chemical compound with the empirical formula AlBrx. Aluminium tribromide is the most common form of aluminium bromide. It is a colorless, sublimable hygroscopic solid; hence old samples tend to be hydrated, mostly as aluminium tribromide hexahydrate (AlBr3·6H2O).

o-Xylene (ortho-xylene) is an aromatic hydrocarbon with the formula C6H4(CH3)2, with two methyl substituents bonded to adjacent carbon atoms of a benzene ring (the ortho configuration). It is a constitutional isomer of m-xylene and p-xylene, the mixture being called xylene or xylenes. o-Xylene is a colourless slightly oily flammable liquid.
Benzyl bromide is an organic compound with the formula C6H5CH2Br. The molecule consists of a benzene ring substituted with a bromomethyl group. It is a colorless liquid with lachrymatory properties. The compound is a reagent for introducing benzyl groups.
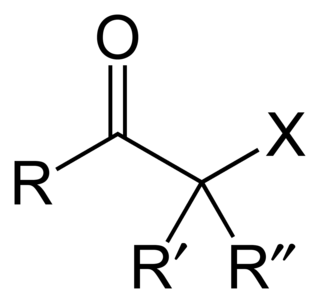
In organic chemistry, an α-halo ketone is a functional group consisting of a ketone group or more generally a carbonyl group with an α-halogen substituent. α-Halo ketones are alkylating agents. Prominent α-halo ketones include phenacyl bromide and chloroacetone.

The Hell–Volhard–Zelinsky halogenation reaction is a chemical transformation that transforms an alkyl carboxylic acid to the α-bromo derivative. It is a specialized and rare kind of halogenation.

Allyl bromide (3-bromopropene) is an organic halide. It is an alkylating agent used in synthesis of polymers, pharmaceuticals, perfumes and other organic compounds. Allyl bromide is a colorless liquid, although commercial samples appear yellow or brown. It is an irritant and a potentially dangerous alkylating agent. Allyl bromide is more reactive but more expensive than allyl chloride, and these considerations guide its use.

Dibromomethane or methylene bromide, or methylene dibromide is a halomethane with the formula CH2Br2. It is slightly soluble in water but very soluble in organic solvents. It is a colorless liquid.

Vinyl bromide is the organobromine compound with the formula CH2=CHBr. Classified as a vinyl halide, it is a colorless gas at room temperature. It is used as a reagent and a comonomer.

Benzyl cyanide (abbreviated BnCN) is an organic compound with the chemical formula C6H5CH2CN. This colorless oily aromatic liquid is an important precursor to numerous compounds in organic chemistry. It is also an important pheromone in certain species.

Phenacyl bromide is the organic compound with the formula C6H5C(O)CH2Br. This colourless solid is a powerful lachrymator as well as a useful precursor to other organic compounds.
Organobromine chemistry is the study of the synthesis and properties of organobromine compounds, also called organobromides, which are organic compounds that contain carbon bonded to bromine. The most pervasive is the naturally produced bromomethane.

In organic chemistry, a xylylene (sometimes quinone-dimethide) is any of the constitutional isomers having the formula C6H4(CH2)2. These compounds are related to the corresponding quinones and quinone methides by replacement of the oxygen atoms by CH2 groups. ortho- and para-xylylene are best known, although neither is stable in solid or liquid form. The meta form is a diradical. Certain substituted derivatives of xylylenes are however highly stable, such as tetracyanoquinodimethane and the xylylene dichlorides.

1-Bromonaphthalene is an organic compound with the formula C10H7Br.

α,α,α',α'-Tetrabromo-o-xylene is an organobromine compound with the formula C6H4(CHBr2)2. Three isomers of α,α,α',α'-Tetrabromoxylene exist, but the ortho derivative is most widely studied. It is an off-white solid. The compound is prepared by the photochemical reaction of o-xylene with elemental bromine:
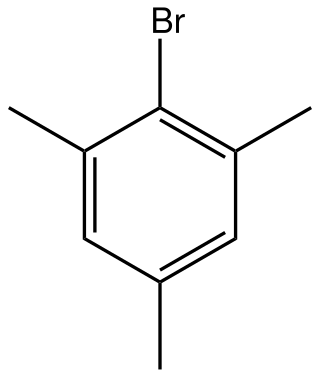
Mesityl bromide is an organic compound with the formula (CH3)3C6H2Br. It is a derivative of mesitylene (1,3,5-trimethylbenzene) with one ring H replaced by Br. The compound is a colorless oil. It is a standard electron-rich aryl halide substrate for cross coupling reactions. With magnesium it reacts to give the Grignard reagent, which is used in the preparation of tetramesityldiiron.
Bromotoluenes are aryl bromides based on toluene in which at least one aromatic hydrogen atom is replaced with a bromine atom. They have the general formula C7H8–nBrn, where n = 1–5 is the number of bromine atoms.

















