
Malonic acid (IUPAC systematic name: propanedioic acid) is a dicarboxylic acid with structure CH2(COOH)2. The ionized form of malonic acid, as well as its esters and salts, are known as malonates. For example, diethyl malonate is malonic acid's diethyl ester. The name originates from the Greek word μᾶλον (malon) meaning 'apple'.

Diethyl malonate, also known as DEM, is the diethyl ester of malonic acid. It occurs naturally in grapes and strawberries as a colourless liquid with an apple-like odour, and is used in perfumes. It is also used to synthesize other compounds such as barbiturates, artificial flavourings, vitamin B1, and vitamin B6.

Amobarbital is a drug that is a barbiturate derivative. It has sedative-hypnotic properties. It is a white crystalline powder with no odor and a slightly bitter taste. It was first synthesized in Germany in 1923. It is considered a short to intermediate acting barbiturate. If amobarbital is taken for extended periods of time, physiological and psychological dependence can develop. Amobarbital withdrawal mimics delirium tremens and may be life-threatening. Amobarbital was manufactured by Eli Lilly and Company in the US under the brand name Amytal in bright blue bullet shaped capsules or pink tablets containing 50, 100, or 200 milligrams of the drug. The drug was also manufactured generically. Amobarbital was widely misused, known as "Blue Heavens" on the street. Amytal, as well as Tuinal, a combination drug containing equal quantities of secobarbital and amobarbital, were both manufactured by Eli Lilly until the late-1990s. However, as the popularity of benzodiazepines increased, prescriptions for these medications became increasingly rare beginning in the mid to late-1980s.

Phenobarbital, also known as phenobarbitone or phenobarb, sold under the brand name Luminal among others, is a medication of the barbiturate type. It is recommended by the World Health Organization (WHO) for the treatment of certain types of epilepsy in developing countries. In the developed world, it is commonly used to treat seizures in young children, while other medications are generally used in older children and adults. In developed countries it is used for veterinary purposes. It may be used intravenously, injected into a muscle, or taken by mouth. The injectable form may be used to treat status epilepticus. Phenobarbital is occasionally used to treat trouble sleeping, anxiety, and drug withdrawal and to help with surgery. It usually begins working within five minutes when used intravenously and half an hour when administered by mouth. Its effects last for between four hours and two days.

In organic chemistry, the Michael reaction or Michael 1,4 addition is a reaction between a Michael donor and a Michael acceptor to produce a Michael adduct by creating a carbon-carbon bond at the acceptor's β-carbon. It belongs to the larger class of conjugate additions and is widely used for the mild formation of carbon-carbon bonds.
Barbituric acid or malonylurea or 6-hydroxyuracil is an organic compound based on a pyrimidine heterocyclic skeleton. It is an odorless powder soluble in water. Barbituric acid is the parent compound of barbiturate drugs, although barbituric acid itself is not pharmacologically active. The compound was first synthesised by Adolf von Baeyer.

Barbital, marketed under the brand names Veronal for the pure acid and Medinal for the sodium salt, was the first commercially available barbiturate. It was used as a sleeping aid (hypnotic) from 1903 until the mid-1950s. The chemical names for barbital are diethylmalonyl urea or diethylbarbituric acid; hence, the sodium salt is known also as sodium diethylbarbiturate.

Methohexital or methohexitone is a drug which is a barbiturate derivative. It is classified as short-acting, and has a rapid onset of action. It is similar in its effects to sodium thiopental, a drug with which it competed in the market for anesthetics.
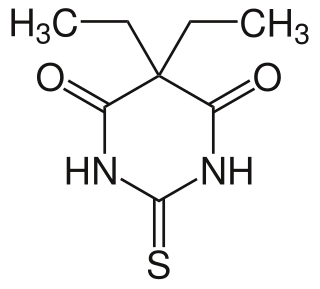
Thiobarbital is a drug which is a barbiturate derivative. It is the thiobarbiturate analogue of barbital.

Josef, Baron von Mering was a German physician.
The malonic ester synthesis is a chemical reaction where diethyl malonate or another ester of malonic acid is alkylated at the carbon alpha to both carbonyl groups, and then converted to a substituted acetic acid.
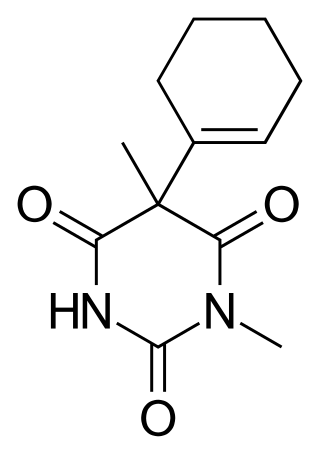
Hexobarbital or hexobarbitone, sold both in acid and sodium salt forms as Citopan, Evipan, and Tobinal, is a barbiturate derivative having hypnotic and sedative effects. It was used in the 1940s and 1950s as an agent for inducing anesthesia for surgery, as well as a rapid-acting, short-lasting hypnotic for general use, and has a relatively fast onset of effects and short duration of action. Modern barbiturates have largely supplanted the use of hexobarbital as an anesthetic, as they allow for better control of the depth of anesthesia. Hexobarbital is still used in some scientific research.

Dimethyl malonate is a diester derivative of malonic acid. It is a common reagent for organic synthesis used, for example, as a precursor for barbituric acid. It is also used in the malonic ester synthesis. It can be synthesized from dimethoxymethane and carbon monoxide.
Barbiturase is a zinc-containing amidohydrolase. Its systemic name is barbiturate amidohydrolase (3-oxo-3-ureidopropanoate-forming). Barbiturase acts as a catalyst in the second step of oxidative pyrimidine degradation, promoting the ring-opening hydrolysis of barbituric acid to ureidomalonic acid. Although grouped into the naturally existing amidohydrolases, it demonstrates more homology with cyanuric acid amidohydrolase. Therefore, it has been proposed that barbiturase, along with cyanuric acid, should be grouped into a new family. KEGG
Dimedone is an organic compound with the formula (CH3)2C(CH2)2(CO)2(CH2). Classified as a cyclic diketone, it is a derivative of 1,3-cyclohexanedione. It is a white solid that is soluble in water, as well as ethanol and methanol. It once was used as a reagent to test for the aldehyde functional group.

Barbiturates are a class of depressant drugs that are chemically derived from barbituric acid. They are effective when used medically as anxiolytics, hypnotics, and anticonvulsants, but have physical and psychological addiction potential as well as overdose potential among other possible adverse effects. They have been used recreationally for their anti-anxiety and sedative effects, and are thus controlled in most countries due to the risks associated with such use.
Within the area of organocatalysis, (thio)urea organocatalysis describes the use of ureas and thioureas to accelerate and stereochemically alter organic transformations. The effects arise through hydrogen-bonding interactions between the substrate and the (thio)urea. Unlike classical catalysts, these organocatalysts interact by non-covalent interactions, especially hydrogen bonding. The scope of these small-molecule H-bond donors termed (thio)urea organocatalysis covers both non-stereoselective and stereoselective applications.

In pharmacology, GABAA receptor positive allosteric modulators, also known as GABAkines or GABAA receptor potentiators, are positive allosteric modulator (PAM) molecules that increase the activity of the GABAA receptor protein in the vertebrate central nervous system.

Diethyl phenylmalonate is an aromatic malonic ester used in the synthesis of moderate to long lasting barbiturates such as phenobarbital.

Diethyl oxomalonate is the diethyl ester of mesoxalic acid (ketomalonic acid), the simplest oxodicarboxylic acid and thus the first member (n = 0) of a homologous series HOOC–CO–(CH2)n–COOH with the higher homologues oxalacetic acid (n = 1), α-ketoglutaric acid (n = 2) and α-ketoadipic acid (n = 3) (the latter a metabolite of the amino acid lysine). Diethyl oxomalonate reacts because of its highly polarized keto group as electrophile in addition reactions and is a highly active reactant in pericyclic reactions such as the Diels-Alder reactions, cycloadditions or ene reactions. At humid air, mesoxalic acid diethyl ester reacts with water to give diethyl mesoxalate hydrate and the green-yellow oil are spontaneously converted to white crystals.
















