
N,N-Dimethyltryptamine is a chemical substance that occurs in many plants and animals and which is both a derivative and a structural analog of tryptamine. It is used as a recreational psychedelic drug and prepared by various cultures for ritual purposes as an entheogen.
Dissociatives are a class of hallucinogen which distort perception of sight and sound and produce feelings of detachment – dissociation – from the environment and/or self. Although many kinds of drugs are capable of such action, dissociatives are unique in that they do so in such a way that they produce hallucinogenic effects, which may include sensory deprivation, dissociation, hallucinations, and dream-like states or trances. Some, which are nonselective in action and affect the dopamine and/or opioid systems, may be capable of inducing euphoria. Many dissociatives have general depressant effects and can produce sedation, respiratory depression, analgesia, anesthesia, and ataxia, as well as cognitive and memory impairment and amnesia.
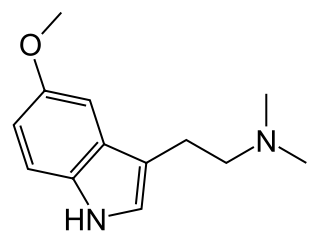
5-MeO-DMT (5-methoxy-N,N-dimethyltryptamine) or O-methyl-bufotenin is a psychedelic of the tryptamine class. It is found in a wide variety of plant species, and at least one toad species, the Sonoran Desert toad. Like its close relatives DMT and bufotenin (5-HO-DMT), it has been used as an entheogen in South America. Slang terms include Five-methoxy, The power, and Toad venom.

N-Ethyltryptamine (NET), or merely ethyltryptamine, is a tryptamine that is structurally related to N-methyltryptamine (NMT) and the psychedelic drugs N,N-dimethyltryptamine (DMT) and N,N-diethyltryptamine (DET).

DET, also known under its chemical name N,N-diethyltryptamine and as T-9, is a psychedelic drug closely related to DMT and 4-HO-DET. However, despite its structural similarity to DMT, its activity is induced by an oral dose of around 50–100 mg, without the aid of MAO inhibitors, and the effects last for about 2–4 hours.

Anadenanthera is a genus of South American trees in the Legume family, Fabaceae. The genus contains two to four species, including A. colubrina and A. peregrina. These trees respectively are known to the western world primarily as sources of the hallucinogenic snuffs Vilca/Cebil and Yopo/Cohoba.

5-MeO-DET or 5-methoxy-N,N-diethyltryptamine is a hallucinogenic tryptamine.

N-Methyltryptamine (NMT) is a member of the substituted tryptamine chemical class and a natural product which is biosynthesized in the human body from tryptamine by certain N-methyltransferase enzymes, such as indolethylamine N-methyltransferase. It is a common component in human urine. NMT is an alkaloid derived from L-tryptophan that has been found in the bark, shoots and leaves of several plant genera, including Virola, Acacia, Mimosa, and Desmanthus—often together with the related compounds N,N-dimethyltryptamine (DMT) and 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT).
The sigma receptorsσ1 and σ2 bind to ligands such as 4-PPBP, SA 4503 (cutamesine), ditolylguanidine, dimethyltryptamine, and siramesine. They are named by pharmacological similarities, and are evolutionarily unrelated.

α,N,N-Trimethyltryptamine is a psychoactive drug of the tryptamine chemical class which acts as a psychedelic hallucinogen. It is similar in structure to the other psychedelics of the tryptamine class such as dimethyltryptamine (DMT) and α-methyltryptamine (α-MT).

5,N,N-trimethyltryptamine is a tryptamine derivative that is a psychedelic drug. It was first made in 1958 by E. H. Young. In animal experiments it was found to be in between DMT and 5-MeO-DMT in potency which would suggest an active dosage for humans in the 20–60 mg range. Human psychoactivity for this compound has been claimed in reports on websites such as Erowid but has not been independently confirmed.

Acacia simplex is a perennial climbing tree native to islands in the western part of the Pacific Ocean as far east as Savaiʻi. It is also found in Argentina. This tree grows up to 12 m in height.

5-MeS-DMT (5-methylthio-N,N-dimethyltryptamine) is a lesser-known psychedelic drug. It is the 5-methylthio analog of dimethyltryptamine (DMT). 5-MeS-DMT was first synthesized by Alexander Shulgin. In his book TiHKAL, the minimum dosage is listed as 15-30 mg. The duration listed as very short, just like DMT. 5-MeS-DMT produces similar effects to DMT, but weaker. Shulgin describes his feelings while on a low dose of this drug as "pointlessly stoned", although at a higher dose of 20 mg he says it is "quite intense" and suggests that a higher dose still might have full activity.

5,6-MDO-DMT, or 5,6-methylenedioxy-N,N-dimethyltryptamine, is a lesser-known psychedelic drug. It is the 5,6-methylenedioxy analog of DMT. 5,6-MDO-DMT was first synthesized by Alexander Shulgin. In his book TiHKAL, 5,6-MDO-DMT produces no noticeable psychoactive effects, although it was only tested up to a dose of 5 mg. Very little data exists about the pharmacological properties, metabolism, and toxicity of 5,6-MDO-DMT.
In enzymology, an amine N-methyltransferase is an enzyme that is ubiquitously present in non-neural tissues and that catalyzes the N-methylation of tryptamine and structurally related compounds.
The molecular formula C12H16N2O (molar mass: 204.26 g/mol, exact mass: 204.126263 u) may refer to:

7,N,N-Trimethyltryptamine (7-methyl-DMT, 7-TMT), is a tryptamine derivative which acts as an agonist of 5-HT2 receptors. In animal tests, both 7-TMT and its 5-methoxy derivative 5-MeO-7-TMT produced behavioural responses similar to those of psychedelic drugs such as DMT, but the larger 7-ethyl and 7-bromo derivatives of DMT did not produce psychedelic responses despite having higher 5-HT2 receptor affinity in vitro (cf. DOBU, DOAM). 7-TMT also weakly inhibits reuptake of serotonin but with little effect on dopamine or noradrenaline reuptake.
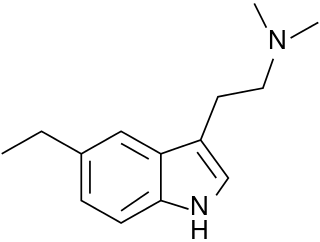
5-Ethyl-N,N-dimethyltryptamine is a tryptamine derivative which acts as an agonist at the 5-HT1A and 5-HT1D serotonin receptors, with around 3x selectivity for 5-HT1D.
α-Endopsychosin is an antagonist of the phencyclidine site of the NMDA receptor which was discovered in extracts of porcine brain and may also be endogenous in humans. The compound appears to be a peptide, but has yet to be purified and fully characterized.















