 | |
| Names | |
|---|---|
| Preferred IUPAC name (Ethenylsulfanyl)ethene | |
| Other names vinyl sulfide, DVS | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
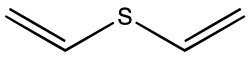
| C4H6S | |
| Molar mass | 86.15 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.9098 g/cm3 (20 °C) |
| Melting point | 20 °C (68 °F; 293 K) |
| Boiling point | 84 °C (183 °F; 357 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Divinyl sulfide is the organosulfur compound with the formula S(CH=CH2)2. A colorless liquid with a faint odor, it is found in some species of Allium . [1]