 | |
 | |
 | |
| Names | |
|---|---|
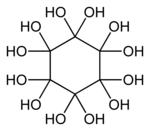
| Preferred IUPAC name Cyclohexanedodecol | |
| Other names Dodecahydroxycyclohexane | |
| Identifiers | |
| |
3D model (JSmol) | |
| ChemSpider | |
PubChem CID | |
| UNII |
|
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| (C(OH)2)6 | |
| Molar mass | 276.150 g·mol−1 |
| Appearance | Colourless crystals (dihydrate) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Dodecahydroxycyclohexane is an organic compound with molecular formula C6O12H12 or C6(OH)12 or (C(OH)2)6. It is a sixfold geminal diol with a cyclohexane backbone and can be regarded as a sixfold hydrate of cyclohexanehexone (C6O6).
