
Alkylation is a chemical reaction that entails transfer of an alkyl group. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene. Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character. In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins. For upgrading of petroleum, alkylation produces a premium blending stock for gasoline. In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents.
In organic chemistry, a carbanion is an anion in which carbon is negatively charged.
In organic chemistry, an aryl halide is an aromatic compound in which one or more hydrogen atoms, directly bonded to an aromatic ring are replaced by a halide. Haloarenes are different from haloalkanes because they exhibit many differences in methods of preparation and properties. The most important members are the aryl chlorides, but the class of compounds is so broad that there are many derivatives and applications.
Cuprates are a class of compounds that contain copper (Cu) atom(s) in an anion. They can be broadly categorized into two main types:

In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure R−CH2−C6H5. Benzyl features a benzene ring attached to a methylene group.
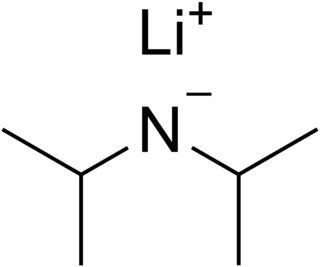
Lithium diisopropylamide is a chemical compound with the molecular formula LiN(CH 2)2. It is used as a strong base and has been widely utilized due to its good solubility in non-polar organic solvents and non-nucleophilic nature. It is a colorless solid, but is usually generated and observed only in solution. It was first prepared by Hamell and Levine in 1950 along with several other hindered lithium diorganylamides to effect the deprotonation of esters at the α position without attack of the carbonyl group.

Ozonide is the polyatomic anion O−3. Cyclic organic compounds formed by the addition of ozone to an alkene are also called ozonides.

Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group [R−N+≡N]X− where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide. The parent compound where R is hydrogen, is diazenylium.

Durene, or 1,2,4,5-tetramethylbenzene, is an organic compound with the formula C6H2(CH3)4. It is a colourless solid with a sweet odor. The compound is classified as an alkylbenzene. It is one of three isomers of tetramethylbenzene, the other two being prehnitene (1,2,3,4-tetramethylbenzene) and isodurene (1,2,3,5-tetramethylbenzene). Durene has an unusually high melting point (79.2 °C), reflecting its high molecular symmetry.

L-selectride is a organoboron compound with the chemical formula Li[(CH3CH2CH )3BH]. A colorless salt, it is usually dispensed as a solution in THF. As a particularly basic and bulky borohydride, it is used for stereoselective reduction of ketones.

Dodecahydroxycyclohexane is an organic compound with molecular formula C6O12H12 or C6(OH)12 or (C 2)6. It is a sixfold geminal diol with a cyclohexane backbone and can be regarded as a sixfold hydrate of cyclohexanehexone.

Decahydroxycyclopentane is an organic compound with formula C5O10H10 or C5(OH)10. It is a fivefold geminal diol on a cyclopentane backbone.

Tetrahydroxy-1,4-benzoquinone, also called tetrahydroxy-p-benzoquinone, tetrahydroxybenzoquinone, or tetrahydroxyquinone, is an organic compound with formula C6O2(OH)4. Its molecular structure consists of a cyclohexadiene ring with four hydroxyl groups and two ketone groups in opposite (para) positions.

Acetylenedicarboxylic acid or butynedioic acid is an organic compound with the formula H2C4O4 or HO−C(=O)−C≡C−C(=O)−OH. It is a crystalline solid that is soluble in diethyl ether.
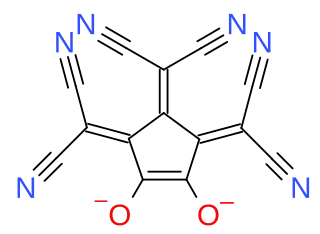
Croconate blue or 1,2,3-tris(dicyanomethylene)croconate is a divalent anion with chemical formula C
14N
6O2−
2 or ((N≡C−)2C=)3(C5O2)2−. It is one of the pseudo-oxocarbon anions, as it can be described as a derivative of the croconate oxocarbon anion C
5O2−
5 through the replacement of three oxygen atoms by dicyanomethylene groups =C(−C≡N)2. The term Croconate Blue as a dye name specifically refers to the dipotassium salt K
2C
14N
6O
2.

Hexamethylbenzene, also known as mellitene, is a hydrocarbon with the molecular formula C12H18 and the condensed structural formula C6(CH3)6. It is an aromatic compound and a derivative of benzene, where benzene's six hydrogen atoms have each been replaced by a methyl group. In 1929, Kathleen Lonsdale reported the crystal structure of hexamethylbenzene, demonstrating that the central ring is hexagonal and flat and thereby ending an ongoing debate about the physical parameters of the benzene system. This was a historically significant result, both for the field of X-ray crystallography and for understanding aromaticity.

Dimethylcadmium is the organocadmium compound with the formula Cd(CH3)2. It is a colorless, highly toxic liquid that fumes in air. It is a linear molecule with C-Cd bond lengths of 213 pm. The compound finds limited use as a reagent in organic synthesis and in metalorganic chemical vapor deposition (MOCVD). It has also been used in the synthesis of cadmium selenide nanoparticles, although efforts have been made to replace it in this capacity due to its toxicity.
Trifluoromethylation in organic chemistry describes any organic reaction that introduces a trifluoromethyl group in an organic compound. Trifluoromethylated compounds are of some importance in pharmaceutical industry and agrochemicals. Several notable pharmaceutical compounds have a trifluoromethyl group incorporated: fluoxetine, mefloquine, Leflunomide, nulitamide, dutasteride, bicalutamide, aprepitant, celecoxib, fipronil, fluazinam, penthiopyrad, picoxystrobin, fluridone, norflurazon, sorafenib and triflurazin. A relevant agrochemical is trifluralin. The development of synthetic methods for adding trifluoromethyl groups to chemical compounds is actively pursued in academic research.
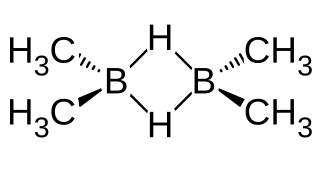
Dimethylborane, (CH3)2BH is the simplest dialkylborane, consisting of a methyl group substituted for a hydrogen in borane. As for other boranes it normally exists in the form of a dimer called tetramethyldiborane or tetramethylbisborane or TMDB ((CH3)2BH)2. Other combinations of methylation occur on diborane, including monomethyldiborane, trimethyldiborane, 1,2-dimethylborane, 1,1-dimethylborane and trimethylborane. At room temperature the substance is at equilibrium between these forms. The methylboranes were first prepared by H. I. Schlesinger and A. O. Walker in the 1930s.
Germyl, trihydridogermanate(1-), trihydrogermanide, trihydridogermyl or according to IUPAC Red Book: germanide is an anion containing germanium bounded with three hydrogens, with formula GeH−3. Germyl is the IUPAC term for the –GeH3 group. For less electropositive elements the bond can be considered covalent rather than ionic as "germanide" indicates. Germanide is the base for germane when it loses a proton.
















