
Energy storage is the capture of energy produced at one time for use at a later time to reduce imbalances between energy demand and energy production. A device that stores energy is generally called an accumulator or battery. Energy comes in multiple forms including radiation, chemical, gravitational potential, electrical potential, electricity, elevated temperature, latent heat and kinetic. Energy storage involves converting energy from forms that are difficult to store to more conveniently or economically storable forms.

Lars Onsager was a Norwegian American physical chemist and theoretical physicist. He held the Gibbs Professorship of Theoretical Chemistry at Yale University. He was awarded the Nobel Prize in Chemistry in 1968.

In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity."

A sodium–sulfur (NaS) battery is a type of molten-salt battery that uses liquid sodium and liquid sulfur electrodes. This type of battery has a similar energy density to lithium-ion batteries, and is fabricated from inexpensive and low-toxicity materials. Due to the high operating temperature required, as well as the highly reactive nature of sodium and sodium polysulfides, these batteries are primarily suited for stationary energy storage applications, rather than for use in vehicles. Molten Na-S batteries are scalable in size: there is a 1 MW microgrid support system on Catalina Island CA (USA) and a 50 MW/300 MWh system in Fukuoka, Kyushu, (Japan). In 2024, only one company produced molten NaS batteries on a commercial scale. BASF Stationary Energy Storage GmbH, a wholly owned subsidiary of BASF SE, acts as a distributor and development partner for the NaS batteries produced by NGK Insulators.

Grid energy storage, also known as large-scale energy storage, are technologies connected to the electrical power grid that store energy for later use. These systems help balance supply and demand by storing excess electricity from variable renewables such as solar and inflexible sources like nuclear power, releasing it when needed. They further provide essential grid services, such as helping to restart the grid after a power outage.

Thermal energy storage (TES) is the storage of thermal energy for later reuse. Employing widely different technologies, it allows surplus thermal energy to be stored for hours, days, or months. Scale both of storage and use vary from small to large – from individual processes to district, town, or region. Usage examples are the balancing of energy demand between daytime and nighttime, storing summer heat for winter heating, or winter cold for summer cooling. Storage media include water or ice-slush tanks, masses of native earth or bedrock accessed with heat exchangers by means of boreholes, deep aquifers contained between impermeable strata; shallow, lined pits filled with gravel and water and insulated at the top, as well as eutectic solutions and phase-change materials.

Electrometallurgy is a method in metallurgy that uses electrical energy to produce metals by electrolysis. It is usually the last stage in metal production and is therefore preceded by pyrometallurgical or hydrometallurgical operations. The electrolysis can be done on a molten metal oxide which is used for example to produce aluminium from aluminium oxide via the Hall-Hérault process. Electrolysis can be used as a final refining stage in pyrometallurgical metal production (electrorefining) and it is also used for reduction of a metal from an aqueous metal salt solution produced by hydrometallurgy (electrowinning).

Molten-salt batteries are a class of battery that uses molten salts as an electrolyte and offers both a high energy density and a high power density. Traditional non-rechargeable thermal batteries can be stored in their solid state at room temperature for long periods of time before being activated by heating. Rechargeable liquid-metal batteries are used for industrial power backup, special electric vehiclesand for grid energy storage, to balance out intermittent renewable power sources such as solar panels and wind turbines.

Molten salt is salt which is solid at standard temperature and pressure but liquified due to elevated temperature. A salt that is liquid even at standard temperature and pressure is usually called a room-temperature ionic liquid, and molten salts are technically a class of ionic liquids.

Daniel George Nocera is an American chemist, currently the Patterson Rockwood Professor of Energy in the Department of Chemistry and Chemical Biology at Harvard University. He is a member of the National Academy of Sciences and the American Academy of Arts and Sciences. In 2006 he was described as a "major force in the field of inorganic photochemistry and photophysics". Time magazine included him in its 2009 list of the 100 most influential people.
Electrochemical engineering is the branch of chemical engineering dealing with the technological applications of electrochemical phenomena, such as electrosynthesis of chemicals, electrowinning and refining of metals, flow batteries and fuel cells, surface modification by electrodeposition, electrochemical separations and corrosion.

Jean-Marie Tarascon FRSC is Professor of Chemistry at the Collège de France in Paris and Director of the French Research Network on Electrochemical Energy Storage (RS2E).
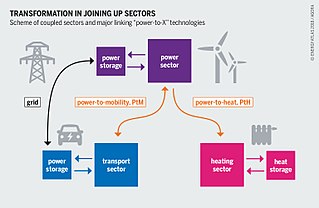
Power-to-X are electricity conversion, energy storage, and reconversion pathways from surplus renewable energy. Power-to-X conversion technologies allow for the decoupling of power from the electricity sector for use in other sectors, possibly using power that has been provided by additional investments in generation. The term is widely used in Germany and may have originated there.
Asegun Sekou Famake Henry is a Robert N. Noyce Career Development Professor in mechanical engineering at Massachusetts Institute of Technology. His research is focused on energy storage, heat transfer, and phonons.

Lynden A. Archer is a chemical engineer, Joseph Silbert Dean of Engineering, David Croll Director of the Energy Systems Institute, and professor of chemical engineering at Cornell University. He became a fellow of the American Physical Society in 2007 and was elected into the National Academy of Engineering in 2018. Archer's research covers polymer and hybrid materials and finds applications in energy storage technologies. His h-index is 92 by Google Scholar.
Ambri Incorporated is an American startup company which aims to produce molten-salt batteries for energy storage in wind and solar power systems. In 2016 it had thirty-seven employees.

Bruno Georges Pollet BSc(Hons) MSc PhD FIAHE FRSC, is an electrochemist and electrochemical engineer, a Fellow of the Royal Society of Chemistry, a Fellow of the International Association for Hydrogen Energy, a full professor of chemistry, director of the Green Hydrogen Lab and member of the Hydrogen Research Institute at the Université du Québec à Trois-Rivières in Canada. He has worked on Hydrogen Energy in the UK, Japan, South Africa, Norway and Canada, and has both industrial and academic experience. He is a prolific scholar, collaborator, and mentor. He is also regarded as one of the most prominent Hydrogen experts and one of the Hydrogen "influencers" in the world.

Toru H. Okabe is a Japanese scientist specializing in materials science, environmental science, resource circulation engineering, and rare metals process engineering, particularly for electronic waste. His most recent work involves the advancement of new processing technology to recycle rare metals like niobium, titanium, yttrium, rhenium, neodymium, other lanthanides and precious metals. He is also involved in sustainable urban mining.

Karim Zaghib is an Algerian-Canadian electrochemist and materials scientist known for his contributions to the field of energy storage and conversion. He is currently Professor of Chemical and Materials Engineering at Concordia University. As former director of research at Hydro-Québec, he helped to make it the world’s first company to use lithium iron phosphate in cathodes, and to develop natural graphite and nanotitanate anodes.
Boston Metal is a company developing a technology known as molten oxide electrolysis (MOE) to decarbonize steelmaking and recover high-value metals from mining waste. The company is based in Woburn, Massachusetts, with its Brazilian subsidiary, Boston Metal do Brasil, based in Coronel Xavier Chaves, Minas Gerais.
















