
Kidney stone disease, also known as renal calculus disease, nephrolithiasis or urolithiasis, is a crystallopathy where a solid piece of material develops in the urinary tract. Renal calculi typically form in the kidney and leave the body in the urine stream. A small calculus may pass without causing symptoms. If a stone grows to more than 5 millimeters, it can cause blockage of the ureter, resulting in sharp and severe pain in the lower back that often radiates downward to the groin. A calculus may also result in blood in the urine, vomiting, or painful urination. About half of people who have had a renal calculus are likely to have another within ten years.

Calcium oxalate (in archaic terminology, oxalate of lime) is a calcium salt of oxalic acid with the chemical formula CaC2O4 or Ca(COO)2. It forms hydrates CaC2O4·nH2O, where n varies from 1 to 3. Anhydrous and all hydrated forms are colorless or white. The monohydrate CaC2O4·H2O occurs naturally as the mineral whewellite, forming envelope-shaped crystals, known in plants as raphides. The two rarer hydrates are dihydrate CaC2O4·2H2O, which occurs naturally as the mineral weddellite, and trihydrate CaC2O4·3H2O, which occurs naturally as the mineral caoxite, are also recognized. Some foods have high quantities of calcium oxalates and can produce sores and numbing on ingestion and may even be fatal. Cultural groups with diets that depend highly on fruits and vegetables high in calcium oxalate, such as those in Micronesia, reduce the level of it by boiling and cooking them. They are a constituent in 76% of human kidney stones. Calcium oxalate is also found in beerstone, a scale that forms on containers used in breweries.

Ultrastructure is the architecture of cells and biomaterials that is visible at higher magnifications than found on a standard optical light microscope. This traditionally meant the resolution and magnification range of a conventional transmission electron microscope (TEM) when viewing biological specimens such as cells, tissue, or organs. Ultrastructure can also be viewed with scanning electron microscopy and super-resolution microscopy, although TEM is a standard histology technique for viewing ultrastructure. Such cellular structures as organelles, which allow the cell to function properly within its specified environment, can be examined at the ultrastructural level.

A lima bean, also commonly known as butter bean, sieva bean, double bean or Madagascar bean, is a legume grown for its edible seeds or beans.

Oxalic acid is an organic acid with the systematic name ethanedioic acid and chemical formula HO−C(=O)−C(=O)−OH, also written as (COOH)2 or (CO2H)2 or H2C2O4. It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name comes from the fact that early investigators isolated oxalic acid from flowering plants of the genus Oxalis, commonly known as wood-sorrels. It occurs naturally in many foods. Excessive ingestion of oxalic acid or prolonged skin contact can be dangerous.
Ergastic substances are non-protoplasmic materials found in cells. The living protoplasm of a cell is sometimes called the bioplasm and distinct from the ergastic substances of the cell. The latter are usually organic or inorganic substances that are products of metabolism, and include crystals, oil drops, gums, tannins, resins and other compounds that can aid the organism in defense, maintenance of cellular structure, or just substance storage. Ergastic substances may appear in the protoplasm, in vacuoles, or in the cell wall.
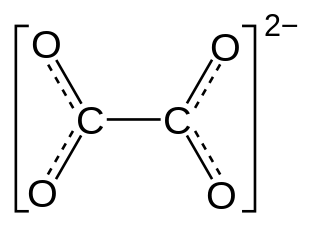
Oxalate is an anion with the chemical formula formula C2O2−4. This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate, and several esters such as dimethyl oxalate. It is a conjugate base of oxalic acid. At neutral pH in aqueous solution, oxalic acid converts completely to oxalate.

Biomineralization, also written biomineralisation, is the process by which living organisms produce minerals, often resulting in hardened or stiffened mineralized tissues. It is an extremely widespread phenomenon: all six taxonomic kingdoms contain members that are able to form minerals, and over 60 different minerals have been identified in organisms. Examples include silicates in algae and diatoms, carbonates in invertebrates, and calcium phosphates and carbonates in vertebrates. These minerals often form structural features such as sea shells and the bone in mammals and birds.

Glyoxylic acid or oxoacetic acid is an organic compound. Together with acetic acid, glycolic acid, and oxalic acid, glyoxylic acid is one of the C2 carboxylic acids. It is a colourless solid that occurs naturally and is useful industrially.

Raphides are needle-shaped crystals of calcium oxalate monohydrate or calcium carbonate as aragonite, found in more than 200 families of plants. Both ends are needle-like, but raphides tend to be blunt at one end and sharp at the other.

Mesembryanthemum tortuosum is a succulent plant in the family Aizoaceae native to the Cape Provinces of South Africa. It is known as kanna, channa, kougoed —which literally means, 'chew(able) things' or 'something to chew'.

Oxalis pes-caprae is a species of tristylous yellow-flowering plant in the wood sorrel family Oxalidaceae. Oxalis cernua is a less common synonym for this species. Some of the most common names for the plant reference its sour taste owing to oxalic acid present in its tissues. Indigenous to South Africa, the plant has become a pest plant in different parts of the world that is difficult to eradicate because of how it propagates through underground bulbs.

Glyoxylate reductase, first isolated from spinach leaves, is an enzyme that catalyzes the reduction of glyoxylate to glycolate, using the cofactor NADH or NADPH.

Tofieldia pusilla is a species of flowering plant in the family Tofieldiaceae. It is also sometimes placed in the lily family, Liliaceae. Its common name is Scottish asphodel in Europe, and Scotch false asphodel in North America. The plant is native to northern North America and parts of Eurasia, its circumpolar distribution extending across Canada and the northern United States to Greenland, Iceland and northern Europe.

Antinutrients are natural or synthetic compounds that interfere with the absorption of nutrients. Nutrition studies focus on antinutrients commonly found in food sources and beverages. Antinutrients may take the form of drugs, chemicals that naturally occur in food sources, proteins, or overconsumption of nutrients themselves. Antinutrients may act by binding to vitamins and minerals, preventing their uptake, or inhibiting enzymes.
Crystal arthropathy is a class of joint disorder that is characterized by accumulation of tiny crystals in one or more joints. Polarizing microscopy and application of other crystallographic techniques have improved identification of different microcrystals including monosodium urate, calcium pyrophosphate dihydrate, calcium hydroxyapatite, and calcium oxalate.
An idioblast is an isolated plant cell that differs from neighboring tissues. They have various functions such as storage of reserves, excretory materials, pigments, and minerals. They could contain oil, latex, gum, resin, tannin, or pigments etc. Some can contain mineral crystals such as acrid tasting and poisonous calcium oxalate, carbonate, or silica. Any of the tissue or tissue systems of plants can contain idioblasts. Idioblasts are divided into three main categories: excretory, tracheoid, and sclerenchymatous.
Chromium(II) oxalate is an inorganic compound with the chemical formula CrC2O4.

Sodium hydrogenoxalate or sodium hydrogen oxalate is a chemical compound with the chemical formula NaHC2O4. It is an ionic compound. It is a sodium salt of oxalic acid H2C2O4. It is an acidic salt, because it consists of sodium cations Na+ and hydrogen oxalate anions HC2O−4 or HO−C(=O)−CO−2, in which only one acidic hydrogen atom in oxalic acid is replaced by sodium atom. The hydrogen oxalate anion can be described as the result of removing one hydrogen ion H+ from oxalic acid, or adding one to the oxalate anion C2O2−4.

Alarm photosynthesis is a variation of photosynthesis where calcium oxalate crystals function as dynamic carbon pools, supplying carbon dioxide (CO2) to photosynthetic cells when stomata are partially or totally closed. This biochemical appendance of the photosynthetic machinery is a means to alleviate the perpetual plant dilemma of using atmospheric CO2 for photosynthesis and losing water vapor, or saving water and reducing photosynthesis. The function of alarm photosynthesis seems to be rather auxiliary to the overall photosynthetic performance. It supports a low photosynthetic rate, aiming at the maintenance and photoprotection of the photosynthetic apparatus rather than a substantial carbon gain.

















