Related Research Articles
An internal ribosome entry site, abbreviated IRES, is an RNA element that allows for translation initiation in a cap-independent manner, as part of the greater process of protein synthesis. In eukaryotic translation, initiation typically occurs at the 5' end of mRNA molecules, since 5' cap recognition is required for the assembly of the initiation complex. The location for IRES elements is often in the 5'UTR, but can also occur elsewhere in mRNAs.
Eukaryotic translation is the biological process by which messenger RNA is translated into proteins in eukaryotes. It consists of four phases: gene regulation, elongation, termination, and recycling.
The Kozak consensus sequence is a nucleic acid motif that functions as the protein translation initiation site in most eukaryotic mRNA transcripts. Regarded as the optimum sequence for initiating translation in eukaryotes, the sequence is an integral aspect of protein regulation and overall cellular health as well as having implications in human disease. It ensures that a protein is correctly translated from the genetic message, mediating ribosome assembly and translation initiation. A wrong start site can result in non-functional proteins. As it has become more studied, expansions of the nucleotide sequence, bases of importance, and notable exceptions have arisen. The sequence was named after the scientist who discovered it, Marilyn Kozak. Kozak discovered the sequence through a detailed analysis of DNA genomic sequences.
Initiation factors are proteins that bind to the small subunit of the ribosome during the initiation of translation, a part of protein biosynthesis.

Ribosome biogenesis is the process of making ribosomes. In prokaryotes, this process takes place in the cytoplasm with the transcription of many ribosome gene operons. In eukaryotes, it takes place both in the cytoplasm and in the nucleolus. It involves the coordinated function of over 200 proteins in the synthesis and processing of the three prokaryotic or four eukaryotic rRNAs, as well as assembly of those rRNAs with the ribosomal proteins. Most of the ribosomal proteins fall into various energy-consuming enzyme families including ATP-dependent RNA helicases, AAA-ATPases, GTPases, and kinases. About 60% of a cell's energy is spent on ribosome production and maintenance.
Eukaryotic initiation factors (eIFs) are proteins or protein complexes involved in the initiation phase of eukaryotic translation. These proteins help stabilize the formation of ribosomal preinitiation complexes around the start codon and are an important input for post-transcription gene regulation. Several initiation factors form a complex with the small 40S ribosomal subunit and Met-tRNAiMet called the 43S preinitiation complex. Additional factors of the eIF4F complex recruit the 43S PIC to the five-prime cap structure of the mRNA, from which the 43S particle scans 5'-->3' along the mRNA to reach an AUG start codon. Recognition of the start codon by the Met-tRNAiMet promotes gated phosphate and eIF1 release to form the 48S preinitiation complex, followed by large 60S ribosomal subunit recruitment to form the 80S ribosome. There exist many more eukaryotic initiation factors than prokaryotic initiation factors, reflecting the greater biological complexity of eukaryotic translation. There are at least twelve eukaryotic initiation factors, composed of many more polypeptides, and these are described below.
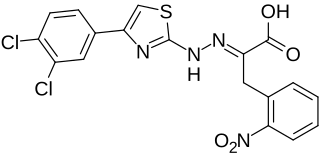
4EGI-1 is a synthetic chemical compound which has been found to interfere with the growth of certain types of cancer cells in vitro. Its mechanism of action involves interruption of the binding of cellular initiation factor proteins involved in the translation of transcribed mRNA at the ribosome. The inhibition of these initiation factors prevents the initiation and translation of many proteins whose functions are essential to the rapid growth and proliferation of cancer cells.

This family represents the internal ribosome entry site (IRES) of the hepatitis A virus. HAV IRES is a 450 nucleotide long sequence located in the 735 nt long 5’ UTR of Hepatitis A viral RNA genome. IRES elements allow cap and end-independent translation of mRNA in the host cell. The IRES achieves this by mediating the internal initiation of translation by recruiting a ribosomal 40S pre-initiation complex directly to the initiation codon and eliminates the requirement for eukaryotic initiation factor, eIF4F.
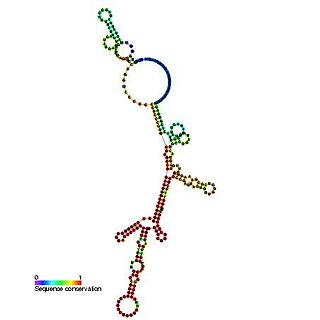
The Hepatitis C virus internal ribosome entry site, or HCV IRES, is an RNA structure within the 5'UTR of the HCV genome that mediates cap-independent translation initiation.
A ribosome binding site, or ribosomal binding site (RBS), is a sequence of nucleotides upstream of the start codon of an mRNA transcript that is responsible for the recruitment of a ribosome during the initiation of translation. Mostly, RBS refers to bacterial sequences, although internal ribosome entry sites (IRES) have been described in mRNAs of eukaryotic cells or viruses that infect eukaryotes. Ribosome recruitment in eukaryotes is generally mediated by the 5' cap present on eukaryotic mRNAs.

Eukaryotic translation initiation factor 4E, also known as eIF4E, is a protein that in humans is encoded by the EIF4E gene.

Eukaryotic translation initiation factor 6 (EIF6), also known as Integrin beta 4 binding protein (ITGB4BP), is a human gene.

Eukaryotic initiation factor 4A-I is a 46 kDa cytosolic protein that, in humans, is encoded by the EIF4A1 gene, which is located on chromosome 17. It is the most prevalent member of the eIF4A family of ATP-dependant RNA helicases, and plays a critical role in the initiation of cap-dependent eukaryotic protein translation as a component of the eIF4F translation initiation complex. eIF4A1 unwinds the secondary structure of RNA within the 5'-UTR of mRNA, a critical step necessary for the recruitment of the 43S preinitiation complex, and thus the translation of protein in eukaryotes. It was first characterized in 1982 by Grifo, et al., who purified it from rabbit reticulocyte lysate.

Eukaryotic translation initiation factor 2A (eIF2A) is a protein that in humans is encoded by the EIF2A gene. The eIF2A protein is not to be confused with eIF2α, a subunit of the heterotrimeric eIF2 complex. Instead, eIF2A functions by a separate mechanism in eukaryotic translation.

Eukaryotic translation initiation factor 1 (eIF1) is a protein that in humans is encoded by the EIF1 gene. It is related to yeast SUI1.
Eukaryotic Initiation Factor 2 (eIF2) is a eukaryotic initiation factor. It is required for most forms of eukaryotic translation initiation. eIF2 mediates the binding of tRNAiMet to the ribosome in a GTP-dependent manner. eIF2 is a heterotrimer consisting of an alpha, a beta, and a gamma subunit.
The eukaryotic initiation factor-4A (eIF4A) family consists of 3 closely related proteins EIF4A1, EIF4A2, and EIF4A3. These factors are required for the binding of mRNA to 40S ribosomal subunits. In addition these proteins are helicases that function to unwind double-stranded RNA.
Translational regulation refers to the control of the levels of protein synthesized from its mRNA. This regulation is vastly important to the cellular response to stressors, growth cues, and differentiation. In comparison to transcriptional regulation, it results in much more immediate cellular adjustment through direct regulation of protein concentration. The corresponding mechanisms are primarily targeted on the control of ribosome recruitment on the initiation codon, but can also involve modulation of peptide elongation, termination of protein synthesis, or ribosome biogenesis. While these general concepts are widely conserved, some of the finer details in this sort of regulation have been proven to differ between prokaryotic and eukaryotic organisms.

Eukaryotic initiation factor 3 (eIF3) is a multiprotein complex that functions during the initiation phase of eukaryotic translation. It is essential for most forms of cap-dependent and cap-independent translation initiation. In humans, eIF3 consists of 13 nonidentical subunits (eIF3a-m) with a combined molecular weight of ~800 kDa, making it the largest translation initiation factor. The eIF3 complex is broadly conserved across eukaryotes, but the conservation of individual subunits varies across organisms. For instance, while most mammalian eIF3 complexes are composed of 13 subunits, budding yeast's eIF3 has only six subunits.

Eukaryotic initiation factor 4F (eIF4F) is a heterotrimeric protein complex that binds the 5' cap of messenger RNAs (mRNAs) to promote eukaryotic translation initiation. The eIF4F complex is composed of three non-identical subunits: the DEAD-box RNA helicase eIF4A, the cap-binding protein eIF4E, and the large "scaffold" protein eIF4G. The mammalian eIF4F complex was first described in 1983, and has been a major area of study into the molecular mechanisms of cap-dependent translation initiation ever since.
References
- ↑ Lodish, Harvey; Berk, Arnold; Kaiser, Chris; Krieger, Monty; Bretscher, Anthony; Ploegh, Hidde; Amon, Angelika; Scott, Matthew. Molecular Cell Biology (7th ed.). New York: W. H. Freeman and Company. ISBN 978-1-4292-3413-9.
- ↑ Goyer C, Altmann M, Lee HS, Blanc A, Deshmukh M, Woolford JL Jr, Trachsel H, Sonenberg N (1993). "TIF4631 and TIF4632: two yeast genes encoding the high-molecular-weight subunits of the cap-binding protein complex (eukaryotic initiation factor 4F) contain an RNA recognition motif-like sequence and carry out an essential function". Molecular and Cellular Biology. 13 (8): 4860–4874. doi:10.1128/MCB.13.8.4860. PMC 360119 . PMID 8336723.
- ↑ Contreras V, Richardson MA, Hao E, Keiper BD (2008). "Depletion of the cap-associated isoform of translation factor eIF4G induces germline apoptosis in C. elegans". Cell Death and Differentiation. 15: 1232–1242. doi: 10.1038/cdd.2008.46 . PMID 18451872.
- ↑ Kally Z. Pan; Julia E. Palter; Aric N. Rogers; Anders Olsen; Di Chen; Gordon J. Lithgow; Pankaj Kapahi (2007). "Inhibition of mRNA translation extends lifespan in Caenorhabditis elegans". Aging Cell. 6 (1): 111–119. doi:10.1111/j.1474-9726.2006.00266.x. PMC 2745345 . PMID 17266680.
- ↑ Rogers AN, Chen D, McColl G, Czerwieniec G, Felkey K, Gibson BW, Hubbard A, Melov S, Lithgow GJ, Kapahi P (2011). "Life span extension via eIF4G inhibition is mediated by posttranscriptional remodeling of stress response gene expression in C. elegans". Cell Metabolism. 14 (1): 55–66. doi:10.1016/j.cmet.2011.05.010. PMC 3220185 . PMID 21723504.
- ↑ Weiniu Gan; Michael La Celle & Robert E. Rhoads (1998). "Functional Characterization of the Internal Ribosome Entry Site of eIF4G mRNA*". The Journal of Biological Chemistry. 273 (9): 5006–5012. doi: 10.1074/jbc.273.9.5006 . PMID 9478948.
- ↑ Ivan B. Lomakin; Christopher U. T. Hellen & Tatyana V. Pestova (2000). "Physical Association of Eukaryotic Initiation Factor 4G (eIF4G) with eIF4A Strongly Enhances Binding of eIF4G to the Internal Ribosomal Entry Site of Encephalomyocarditis Virus and Is Required for Internal Initiation of Translation". Mol Cell Biol. 20 (16): 6019–6029. doi:10.1128/MCB.20.16.6019-6029.2000. PMC 86078 . PMID 10913184.