
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen, sulfur and, more rarely, other elements such as phosphorus, chlorine, and bromine.

Aconitine is an alkaloid toxin produced by various plant species belonging to the genus Aconitum, known also commonly by the names wolfsbane and monkshood. Monkshood is notorious for its toxic properties.
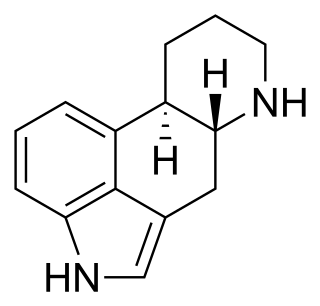
Ergoline is a chemical compound whose structural skeleton is contained in a variety of alkaloids, referred to as ergoline derivatives or ergoline alkaloids. Ergoline alkaloids, one being ergine, were initially characterized in ergot. Some of these are implicated in the condition ergotism, which can take a convulsive form or a gangrenous form. Even so, many ergoline alkaloids have been found to be clinically useful. Annual world production of ergot alkaloids has been estimated at 5,000–8,000 kg of all ergopeptines and 10,000–15,000 kg of lysergic acid, used primarily in the manufacture of semi-synthetic derivatives.

Papaver somniferum, commonly known as the opium poppy or breadseed poppy, is a species of flowering plant in the family Papaveraceae. It is the species of plant from which both opium and poppy seeds are derived and is also a valuable ornamental plant, grown in gardens. Its native range is probably the eastern Mediterranean, but is now obscured by ancient introductions and cultivation, being naturalized across much of Europe and Asia.

Noscapine is a benzylisoquinoline alkaloid, of the phthalideisoquinoline structural subgroup, which has been isolated from numerous species of the family Papaveraceae. It lacks significant hypnotic, euphoric, or analgesic effects affording it with very low addictive potential. This agent is primarily used for its antitussive (cough-suppressing) effects.

Argemone mexicana is a species of poppy found in Mexico and now widely naturalized in many parts of the world. An extremely hardy pioneer plant, it is tolerant of drought and poor soil, often being the only cover on new road cuttings or verges. It has bright yellow latex. It is poisonous to grazing animals, and it is rarely eaten, but it has been used medicinally by many peoples, including those in its native area, as well as the Natives of the western US, parts of Mexico and many parts of India. In India, during the colorful festival Holika Dahan, adults and children worship by offering flowers, and this species is in its maximum flowering phase during March when the Holi festival is celebrated. It is also referred to as "kateli ka phool” in India.

Catharanthus roseus, commonly known as bright eyes, Cape periwinkle, graveyard plant, Madagascar periwinkle, old maid, pink periwinkle, rose periwinkle, is a perennial species of flowering plant in the family Apocynaceae. It is native and endemic to Madagascar, but grown elsewhere as an ornamental and medicinal plant. It is a source of the drugs vincristine and vinblastine, used to treat cancer. It was formerly included in the genus Vinca as Vinca rosea.

Ephedra is a genus of gymnosperm shrubs. The various species of Ephedra are widespread in many arid regions of the world, ranging across southwestern North America, southern Europe, northern Africa, southwest and central Asia, northern China and western South America. It is the only extant genus in its family, Ephedraceae, and order, Ephedrales, and one of the three living members of the division Gnetophyta alongside Gnetum and Welwitschia.

Eupatorium cannabinum, commonly known as hemp-agrimony, or holy rope, is a herbaceous plant in the family Asteraceae. It is a robust perennial native to Europe, NW. Africa, Turkey, Syria, Iran, Iraq, Jordan, the Caucasus and Central Asia. It is cultivated as an ornamental and occasionally found as a garden escape in scattered locations in China, the United States and Canada. It is extremely attractive to butterflies, much like buddleia.

Conessine is a steroidal alkaloid found in a number of plant species from the family Apocynaceae, including Holarrhena floribunda, Holarrhena antidysenterica and Funtumia elastica. It acts as a histamine antagonist, selective for the H3 subtype (with an affinity of pKi = 8.27; Ki = ~5 nM). It was also found to have long CNS clearance times, high blood–brain barrier penetration and high affinity for the adrenergic receptors.

Tetrandrine, a bis-benzylisoquinoline alkaloid, is a calcium channel blocker. It is isolated from the plant Stephania tetrandra, and other Chinese and Japanese herbs.

Solasodine is a poisonous alkaloid chemical compound that occurs in plants of the family Solanaceae such as potatoes and tomatoes. Solasonine and solamargine are glycoalkaloid derivatives of solasodine. Solasodine is teratogenic to hamster fetuses in a dose of 1200 to 1600 mg/kg. Literature survey reveals that solasodine has diuretic, anticancer, antifungal, cardiotonic, antispermatogenetic, antiandrogenic, immunomodulatory, antipyretic and various effects on central nervous system.
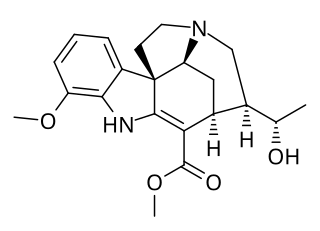
Scholarine is an alkaloid isolated initially from Alstonia scholaris by Banerji and Siddhanta. Subsequently, it was obtained from the West African tree Alstonia boonei. The derivative N-Formylscholarine has been isolated from the fruit pods of Alstonia scholaris.

Darienine is an anti-cholinergic alkaloid.
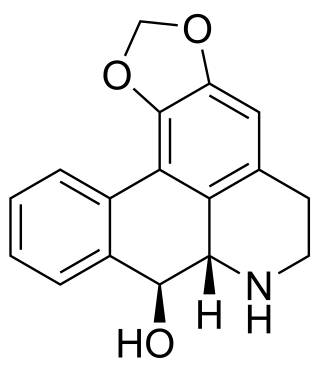
Noroliveroline is an anticholinergic alkaloid.
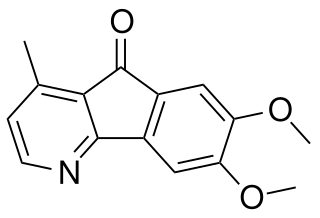
Polyfothine is an anticholinergic alkaloid.

Eburnamenine is an anticholinergic alkaloid.
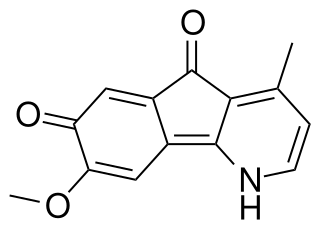
Isooncodine is an anticholinergic alkaloid. It was first synthesized in 1989 because it is an isomer of oncodine, an azafluorenone alkaloid derived from Meiogyne monosperma. It was first derived from the leaves of Polyalthia longifolia.
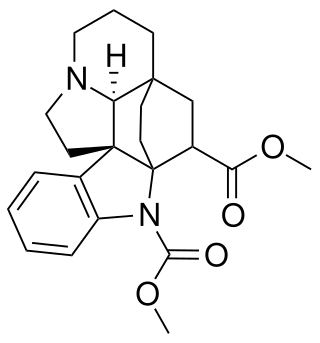
Pleiocarpine is an anticholinergic alkaloid.
Dewan Singh Bhakuni is an Indian natural product chemist, stereochemist and a former director general-grade scientist of the Central Drug Research Institute. He is known for his researches on the biogenesis of alkaloids and is an elected fellow of the Indian Academy of Sciences, the National Academy of Sciences, India and the Indian National Science Academy. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards, in 1975, for his contributions to chemical sciences.



















