
Fusarium ear blight (FEB), is a fungal disease of cereals, including wheat, barley, oats, rye and triticale. FEB is caused by a range of Fusarium fungi, which infects the heads of the crop, reducing grain yield. The disease is often associated with contamination by mycotoxins produced by the fungi already when the crop is growing in the field. The disease can cause severe economic losses as mycotoxin-contaminated grain cannot be sold for food or feed.

Qo inhibitors (QoI), or quinone outside inhibitors, are a group of fungicides used in agriculture. Some of these fungicides are among the most popular in the world. QoI are chemical compounds which act at the quinol outer binding site of the cytochrome bc1 complex.

Famoxadone is a fungicide to protect agricultural products against various fungal diseases on fruiting vegetables, tomatoes, potatoes, curcurbits, lettuce and grapes. It is used in combination with cymoxanil. Famoxadone is a QoI, albeit with a chemistry different from most QoIs. It is commonly used against Plasmopara viticola, Alternaria solani, Phytophthora infestans, and Septoria nodorum.
Fungicides are pesticides used to kill parasitic fungi or their spores. Fungi can cause serious damage in agriculture, resulting in losses of yield and quality. Fungicides are used both in agriculture and to fight fungal infections in animals. Fungicides are also used to control oomycetes, which are not taxonomically/genetically fungi, although sharing similar methods of infecting plants. Fungicides can either be contact, translaminar or systemic. Contact fungicides are not taken up into the plant tissue and protect only the plant where the spray is deposited. Translaminar fungicides redistribute the fungicide from the upper, sprayed leaf surface to the lower, unsprayed surface. Systemic fungicides are taken up and redistributed through the xylem vessels. Few fungicides move to all parts of a plant. Some are locally systemic, and some move upward.

The smuts are multicellular fungi characterized by their large numbers of teliospores. The smuts get their name from a Germanic word for 'dirt' because of their dark, thick-walled, and dust-like teliospores. They are mostly Ustilaginomycetes and comprise seven of the 15 orders of the subphylum. Most described smuts belong to two orders, Ustilaginales and Tilletiales. The smuts are normally grouped with the other basidiomycetes because of their commonalities concerning sexual reproduction.
A Biopesticide is a biological substance or organism that damages, kills, or repels organisms seens as pests. Biological pest management intervention involves predatory, parasitic, or chemical relationships.
A triazole is a heterocyclic compound featuring a five-membered ring of two carbon atoms and three nitrogen atoms with molecular formula C2H3N3. Triazoles exhibit substantial isomerism, depending on the positioning of the nitrogen atoms within the ring.
The cereal grain wheat is subject to numerous wheat diseases, including bacterial, viral and fungal diseases, as well as parasitic infestations.

Fusarium culmorum is a fungal plant pathogen and the causal agent of seedling blight, foot rot, ear blight, stalk rot, common root rot and other diseases of cereals, grasses, and a wide variety of monocots and dicots. In coastal dunegrass, F. culmorum is a nonpathogenic symbiont conferring both salt and drought tolerance to the plant.

Zymoseptoria tritici, synonyms Septoria tritici, Mycosphaerella graminicola, is a species of filamentous fungus, an ascomycete in the family Mycosphaerellaceae. It is a wheat plant pathogen causing septoria leaf blotch that is difficult to control due to resistance to multiple fungicides. The pathogen today causes one of the most important diseases of wheat.

Ascochyta is a genus of ascomycete fungi, containing several species that are pathogenic to plants, particularly cereal crops. The taxonomy of this genus is still incomplete. The genus was first described in 1830 by Marie-Anne Libert, who regarded the spores as minute asci and the cell contents as spherical spores. Numerous revisions to the members of the genus and its description were made for the next several years. Species that are plant pathogenic on cereals include, A. hordei, A. graminea, A. sorghi, A. tritici. Symptoms are usually elliptical spots that are initially chlorotic and later become a necrotic brown. Management includes fungicide applications and sanitation of diseased plant tissue debris.
Septoria cannabis is a species of plant pathogen from the genus Septoria that causes the disease commonly known as Septoria leaf spot. Early symptoms of infection are concentric white lesions on the vegetative leaves of cannabis plants, followed by chlorosis and necrosis of the leaf until it is ultimately overcome by disease and all living cells are then killed. Septoria, which is an ascomycete and pycnidia producing fungus, has been well known to attack Solanaceae and Cucurbitaceae species as well as many tree species. This genus is known to comprise over 1,000 species of pathogens, each infecting a specific and unique host.
Septoria secalis also known as Septoria Leaf Blotch is a fungal plant pathogen infecting rye.

Azoxystrobin is a broad spectrum systemic fungicide widely used in agriculture to protect crops from fungal diseases. It was first marketed in 1996 using the brand name Amistar and by 1999 it had been registered in 48 countries on more than 50 crops. In the year 2000 it was announced that it had been granted UK Millennium product status.
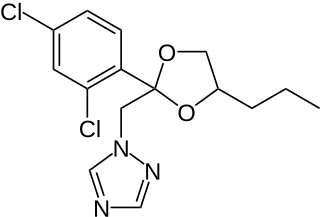
Propiconazole is a triazole fungicide, also known as a DMI, or demethylation inhibiting fungicide due to its binding with and inhibiting the 14-alpha demethylase enzyme from demethylating a precursor to ergosterol. Without this demethylation step, the ergosterols are not incorporated into the growing fungal cell membranes, and cellular growth is stopped.
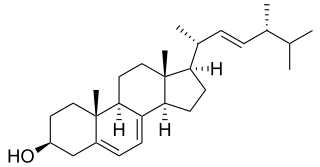
In enzymology, a sterol 14-demethylase (EC 1.14.13.70) is an enzyme of the cytochrome P450 (CYP) superfamily. It is any member of the CYP51 family. It catalyzes a chemical reaction such as:

Cyproconazole is an agricultural fungicide of the class of azoles, used on cereal crops, coffee, sugar beet, fruit trees and grapes, and peanuts, on sod farms and golf course turf and on wood as a preservative. It has been used against powdery mildew, rust on cereals and apple scab, and applied by air or on the ground or by chemigation.
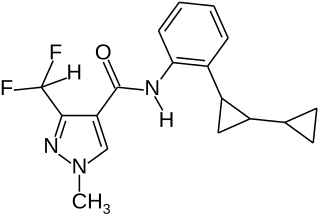
Sedaxane is a broad spectrum fungicide used as a seed treatment in agriculture to protect crops from fungal diseases. It was first marketed by Syngenta in 2011 using their brand name Vibrance. The compound is an amide which combines a pyrazole acid with an aryl amine to give an inhibitor of succinate dehydrogenase.

Prochloraz, brand name Sportak, is an imidazole fungicide that was introduced in 1978 and is widely used in Europe, Australia, Asia, and South America within gardening and agriculture to control the growth of fungi. It is not registered for use in the United States. Similarly to other azole fungicides, prochloraz is an inhibitor of the enzyme lanosterol 14α-demethylase (CYP51A1), which is necessary for the production of ergosterol – an essential component of the fungal cell membrane – from lanosterol. The agent is a broad-spectrum, protective and curative fungicide, effective against Alternaria spp., Botrytis spp., Erysiphe spp., Helminthosporium spp., Fusarium spp., Pseudocerosporella spp., Pyrenophora spp., Rhynchosporium spp., and Septoria spp.

Pydiflumetofen is a broad spectrum fungicide used in agriculture to protect crops from fungal diseases. It was first marketed by Syngenta in 2016 using their brand name Miravis. The compound is an amide which combines a pyrazole acid with a substituted phenethylamine to give an inhibitor of succinate dehydrogenase, an enzyme that inhibits cellular respiration in almost all living organisms.











