
Estrone (E1), also spelled oestrone, is a steroid, a weak estrogen, and a minor female sex hormone. It is one of three major endogenous estrogens, the others being estradiol and estriol. Estrone, as well as the other estrogens, are synthesized from cholesterol and secreted mainly from the gonads, though they can also be formed from adrenal androgens in adipose tissue. Relative to estradiol, both estrone and estriol have far weaker activity as estrogens. Estrone can be converted into estradiol, and serves mainly as a precursor or metabolic intermediate of estradiol. It is both a precursor and metabolite of estradiol.
Hot flashes are a form of flushing, often caused by the changing hormone levels that are characteristic of menopause. They are typically experienced as a feeling of intense heat with sweating and rapid heartbeat, and may typically last from two to 30 minutes for each occurrence.

Selective estrogen receptor modulators (SERMs), also known as estrogen receptor agonists/antagonists (ERAAs), are a class of drugs that act on estrogen receptors (ERs). Compared to pure ER agonists–antagonists, SERMs are more tissue-specific, allowing them to selectively inhibit or stimulate estrogen-like action in various tissues.
Fulvestrant, sold under the brand name Faslodex among others, is an antiestrogenic medication used to treat hormone receptor (HR)-positive metastatic breast cancer in postmenopausal women with disease progression as well as HR-positive, HER2-negative advanced breast cancer in combination with abemaciclib or palbociclib in women with disease progression after endocrine therapy. It is given by injection into a muscle.

Equol (4',7-isoflavandiol) is an isoflavandiol estrogen metabolized from daidzein, a type of isoflavone found in soybeans and other plant sources, by bacterial flora in the intestines. While endogenous estrogenic hormones such as estradiol are steroids, equol is a nonsteroidal estrogen. Only about 30–50% of people have intestinal bacteria that make equol.

Estrogen receptors (ERs) are proteins found in cells that function as receptors for the hormone estrogen (17β-estradiol). There are two main classes of ERs. The first includes the intracellular estrogen receptors, namely ERα and ERβ, which belong to the nuclear receptor family. The second class consists of membrane estrogen receptors (mERs), such as GPER (GPR30), ER-X, and Gq-mER, which are primarily G protein-coupled receptors. This article focuses on the nuclear estrogen receptors.

Raloxifene, sold under the brand name Evista among others, is a medication used to prevent and treat osteoporosis in postmenopausal women and those on glucocorticoids. For osteoporosis it is less preferred than bisphosphonates. It is also used to reduce the risk of breast cancer in those at high risk. It is taken by mouth.

Tibolone, sold under the brand name Livial among others, is a medication which is used in menopausal hormone therapy and in the treatment of postmenopausal osteoporosis and endometriosis. The medication is available alone and is not formulated or used in combination with other medications. It is taken by mouth.

Estrogen receptor beta (ERβ) also known as NR3A2 is one of two main types of estrogen receptor—a nuclear receptor which is activated by the sex hormone estrogen. In humans ERβ is encoded by the ESR2 gene.
Antiestrogens, also known as estrogen antagonists or estrogen blockers, are a class of drugs which prevent estrogens like estradiol from mediating their biological effects in the body. They act by blocking the estrogen receptor (ER) and/or inhibiting or suppressing estrogen production. Antiestrogens are one of three types of sex hormone antagonists, the others being antiandrogens and antiprogestogens. Antiestrogens are commonly used to stop steroid hormones, estrogen, from binding to the estrogen receptors leading to the decrease of estrogen levels. Decreased levels of estrogen can lead to complications in sexual development.

Coumestrol is a natural organic compound in the class of phytochemicals known as coumestans. Coumestrol was first identified as a compound with estrogenic properties by E. M. Bickoff in ladino clover and alfalfa in 1957. It has garnered research interest because of its estrogenic activity and prevalence in some foods, including soybeans, brussels sprouts, spinach and a variety of legumes. The highest concentrations of coumestrol are found in clover, Kala Chana, and Alfalfa sprouts.
Hormone replacement therapy (HRT), also known as menopausal hormone therapy or postmenopausal hormone therapy, is a form of hormone therapy used to treat symptoms associated with female menopause. Effects of menopause can include symptoms such as hot flashes, accelerated skin aging, vaginal dryness, decreased muscle mass, and complications such as osteoporosis, sexual dysfunction, and vaginal atrophy. They are mostly caused by low levels of female sex hormones that occur during menopause.
Menerba, also known as Menopause Formula 101 (MF-101), is a botanical drug candidate that acts as a selective estrogen receptor modulator (SERM) which is being studied for its potential to relieve hot flashes associated with menopause. Menerba, an estrogen receptor beta (ERβ) agonist (ERBA), is part of a new class of receptor subtype-selective estrogens, which is selective in transcriptional regulation to one of the two known estrogen receptor (ER) subtypes. Menerba consists of 22 herbs that have been used historically in traditional Chinese medicine.

8-Prenylnaringenin (8-PN; also known as flavaprenin, (S)-8-dimethylallylnaringenin, hopein, or sophoraflavanone B) is a prenylflavonoid phytoestrogen. It is reported to be the most estrogenic phytoestrogen known. The compound is equipotent at the two forms of estrogen receptors, ERα and ERβ, and it acts as a full agonist of ERα. Its effects are similar to those of estradiol, but it is considerably less potent in comparison.

A nonsteroidal estrogen is an estrogen with a nonsteroidal chemical structure. The most well-known example is the stilbestrol estrogen diethylstilbestrol (DES). Although nonsteroidal estrogens formerly had an important place in medicine, they have gradually fallen out of favor following the discovery of toxicities associated with high-dose DES starting in the early 1970s, and are now almost never used. On the other hand, virtually all selective estrogen receptor modulators (SERMs) are nonsteroidal, with triphenylethylenes like tamoxifen and clomifene having been derived from DES, and these drugs remain widely used in medicine for the treatment of breast cancer among other indications. In addition to pharmaceutical drugs, many xenoestrogens, including phytoestrogens, mycoestrogens, and synthetic endocrine disruptors like bisphenol A, are nonsteroidal substances with estrogenic activity.
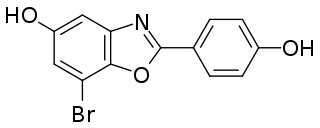
WAY-200070 is a synthetic, nonsteroidal, highly selective agonist of ERβ. It possesses 68-fold selectivity for ERβ over ERα (EC50 = 2 nM and 155 nM, respectively). WAY-200070 has been found to enhance serotonergic and dopaminergic neurotransmission in the central nervous system, and produces antidepressant- and anxiolytic-like effects in animals. It has been proposed as a potential novel antidepressant/anxiolytic agent. WAY-200070 has also been found to produce antidiabetic effects in animals, and may also be beneficial for the treatment of certain inflammatory conditions.

Endoxifen, also known as 4-hydroxy-N-desmethyltamoxifen, is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group as well as a protein kinase C (PKC) inhibitor. It is under development for the treatment of estrogen receptor-positive breast cancer and for the treatment of mania in bipolar disorder. It is taken by mouth.
ERA-45 is a synthetic estrogen and a selective agonist of the ERα. It shows 286-fold selectivity for transactivation of the ERα over the ERβ, with EC50 values of 0.37 nM for the ERα (7-fold weaker than estradiol) and 13 nM for the ERβ (20,000-fold weaker than estradiol). However, another found only about 35-fold potency for transactivation of the ERα over the ERβ. The drug has no antagonistic activity at either receptor. ERA-45 induced prostate cancer development in preclinical models when it was given in combination with testosterone, whereas testosterone alone did not do so. In contrast, the selective ERβ agonist ERB-26 was protective against the development of prostate cancer produced by these two drugs. These findings suggest opposing roles of the ERα and ERβ in the prostate gland. The chemical structure of ERa-45 does not appear to have been disclosed.

An estrogen (E) is a type of medication which is used most commonly in hormonal birth control and menopausal hormone therapy, and as part of feminizing hormone therapy for transgender women. They can also be used in the treatment of hormone-sensitive cancers like breast cancer and prostate cancer and for various other indications. Estrogens are used alone or in combination with progestogens. They are available in a wide variety of formulations and for use by many different routes of administration. Examples of estrogens include bioidentical estradiol, natural conjugated estrogens, synthetic steroidal estrogens like ethinylestradiol, and synthetic nonsteroidal estrogens like diethylstilbestrol. Estrogens are one of three types of sex hormone agonists, the others being androgens/anabolic steroids like testosterone and progestogens like progesterone.
The pharmacology of estradiol, an estrogen medication and naturally occurring steroid hormone, concerns its pharmacodynamics, pharmacokinetics, and various routes of administration.
















