
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at 114 degrees Celsius, and boils to a violet gas at 184 degrees Celsius. The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek Ιώδης 'violet-coloured'.

Tincture of iodine, iodine tincture, or weak iodine solution is an antiseptic. It is usually 2 to 7% elemental iodine, along with potassium iodide or sodium iodide, dissolved in a mixture of ethanol and water. Tincture solutions are characterized by the presence of alcohol. It was used from 1908 in pre-operative skin preparation by surgeon Antonio Grossich.
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency affects two billion people and is the leading preventable cause of intellectual disability.

Potassium iodide is a chemical compound, medication, and dietary supplement. As a medication it is used to treat hyperthyroidism, in radiation emergencies, and to protect the thyroid gland when certain types of radiopharmaceuticals are used. In the developing world it is also used to treat skin sporotrichosis and phycomycosis. As a supplement it is used in those who have low intake of iodine in the diet. It is administered orally.
Micronutrients are essential dietary elements required by organisms in varying quantities throughout life to orchestrate a range of physiological functions to maintain health. Micronutrient requirements differ between organisms; for example, humans and other animals require numerous vitamins and dietary minerals, whereas plants require specific minerals. For human nutrition, micronutrient requirements are in amounts generally less than 100 milligrams per day, whereas macronutrients are required in gram quantities daily.

Iodised salt is table salt mixed with a minute amount of various salts of the element iodine. The ingestion of iodine prevents iodine deficiency. Worldwide, iodine deficiency affects about two billion people and is the leading preventable cause of intellectual and developmental disabilities. Deficiency also causes thyroid gland problems, including endemic goitre. In many countries, iodine deficiency is a major public health problem that can be cheaply addressed by purposely adding small amounts of iodine to the sodium chloride salt.

Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quantities in seawater. The open ocean has about 35 g (1.2 oz) of solids per liter of sea water, a salinity of 3.5%.
Iodine-131 is an important radioisotope of iodine discovered by Glenn Seaborg and John Livingood in 1938 at the University of California, Berkeley. It has a radioactive decay half-life of about eight days. It is associated with nuclear energy, medical diagnostic and treatment procedures, and natural gas production. It also plays a major role as a radioactive isotope present in nuclear fission products, and was a significant contributor to the health hazards from open-air atomic bomb testing in the 1950s, and from the Chernobyl disaster, as well as being a large fraction of the contamination hazard in the first weeks in the Fukushima nuclear crisis. This is because 131I is a major fission product of uranium and plutonium, comprising nearly 3% of the total products of fission. See fission product yield for a comparison with other radioactive fission products. 131I is also a major fission product of uranium-233, produced from thorium.
Iodine deficiency is a lack of the trace element iodine, an essential nutrient in the diet. It may result in metabolic problems such as goiter, sometimes as an endemic goiter as well as congenital iodine deficiency syndrome due to untreated congenital hypothyroidism, which results in developmental delays and other health problems. Iodine deficiency is an important global health issue, especially for fertile and pregnant women. It is also a preventable cause of intellectual disability.

An iodate is the anion with the formula IO−
3. It is the most common form of iodine in nature, as it comprises the major iodine-containing ores. Iodate salts are often colorless. They are the salts of iodic acid.

Iodic acid, is a white water-soluble solid with the chemical formula HIO3. Its robustness contrasts with the instability of chloric acid and bromic acid. Iodic acid features iodine in the oxidation state +5 and is one of the most stable oxo-acids of the halogens. When heated, samples dehydrate to give iodine pentoxide. On further heating, the iodine pentoxide further decomposes, giving a mix of iodine, oxygen and lower oxides of iodine.

The iodine clock reaction is a classical chemical clock demonstration experiment to display chemical kinetics in action; it was discovered by Hans Heinrich Landolt in 1886. The iodine clock reaction exists in several variations, which each involve iodine species and redox reagents in the presence of starch. Two colourless solutions are mixed and at first there is no visible reaction. After a short time delay, the liquid suddenly turns to a shade of dark blue due to the formation of a triiodide–starch complex. In some variations, the solution will repeatedly cycle from colorless to blue and back to colorless, until the reagents are depleted.
Food fortification or enrichment is the process of adding micronutrients to food. It can be carried out by food manufacturers, or by governments as a public health policy which aims to reduce the number of people with dietary deficiencies within a population. The predominant diet within a region can lack particular nutrients due to the local soil or from inherent deficiencies within the staple foods; the addition of micronutrients to staples and condiments can prevent large-scale deficiency diseases in these cases.

Potassium iodate (KIO3) is an ionic chemical compound consisting of K+ ions and IO3− ions in a 1:1 ratio.

Sodium iodate (NaIO3) is the sodium salt of iodic acid. Sodium iodate is an oxidizing agent, and as such it can cause fires upon contact with combustible materials or reducing agents.
Calcium iodates are inorganic compound composed of calcium and iodate anion. Two forms are known, anhydrous Ca(IO3)2 and the monohydrate Ca(IO3)2(H2O). Both are colourless salts that occur naturally as the minerals called lautarite and bruggenite, respectively. A third mineral form of calcium iodate is dietzeite, a salt containing chromate with the formula Ca2(IO3)2CrO4.
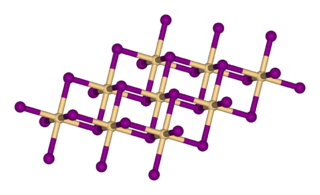
Calcium iodide (chemical formula CaI2) is the ionic compound of calcium and iodine. This colourless deliquescent solid is a salt that is highly soluble in water. Its properties are similar to those for related salts, such as calcium chloride. It is used in photography. It is also used in cat food as a source of iodine.
Iodine deficiency is a widespread problem in western, southern and eastern parts of China, as their iodized salt intake level is much lower than the average national level. Iodine deficiency is a range of disorders that affect many different populations. It is estimated that IDDs affect between 800 million and 2 billion people worldwide; countries have spent millions of dollars in implementing iodized salt as a means to counteract the iodine deficiencies prevalent today. With China accounting for "40% of the total population", it bears a large portion of those who are iodine deficient.
Iodine is an essential trace element in biological systems. It has the distinction of being the heaviest element commonly needed by living organisms as well as the second-heaviest known to be used by any form of life. It is a component of biochemical pathways in organisms from all biological kingdoms, suggesting its fundamental significance throughout the evolutionary history of life.
A feed additive is an additive of extra nutrient or drug for livestock. Such additives include vitamins, amino acids, fatty acids, minerals, pharmaceutical, fungal products and steroidal compounds. The additives might impact feed presentation, hygiene, digestibility, or effect on intestinal health.










