Diffusing capacity of the lung (DL) measures the transfer of gas from air in the lung, to the red blood cells in lung blood vessels. It is part of a comprehensive series of pulmonary function tests to determine the overall ability of the lung to transport gas into and out of the blood. DL, especially DLCO, is reduced in certain diseases of the lung and heart. DLCO measurement has been standardized according to a position paper by a task force of the European Respiratory and American Thoracic Societies.

Furosemide, sold under the brand name Lasix among others, is a loop diuretic medication used to treat edema due to heart failure, liver scarring, or kidney disease. Furosemide may also be used for the treatment of high blood pressure. It can be taken intravenously or orally. When given intravenously, furosemide typically takes effect within five minutes; when taken orally, it typically metabolizes within an hour.
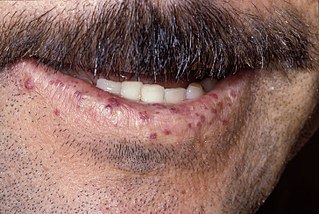
Hereditary hemorrhagic telangiectasia (HHT), also known as Osler–Weber–Rendu disease and Osler–Weber–Rendu syndrome, is a rare autosomal dominant genetic disorder that leads to abnormal blood vessel formation in the skin, mucous membranes, and often in organs such as the lungs, liver, and brain.
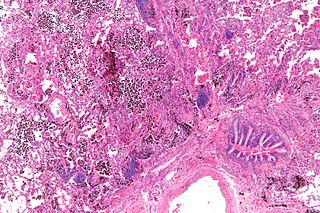
Pulmonary hemorrhage is an acute bleeding from the lung, from the upper respiratory tract and the trachea, and the pulmonary alveoli. When evident clinically, the condition is usually massive. The onset of pulmonary hemorrhage is characterized by a cough productive of blood (hemoptysis) and worsening of oxygenation leading to cyanosis. Treatment should be immediate and should include tracheal suction, oxygen, positive pressure ventilation, and correction of underlying abnormalities such as disorders of coagulation. A blood transfusion may be necessary.

Flunixin is a nonsteroidal anti-inflammatory drug (NSAID), analgesic, and antipyretic used in horses, cattle and pigs. It is often formulated as the meglumine salt. In the United States, it is regulated by the U.S. Food and Drug Administration (FDA), and may only be lawfully distributed by order of a licensed veterinarian. There are many trade names for the product.
Cardiac asthma is the medical condition of intermittent wheezing, coughing, and shortness of breath that is associated with underlying congestive heart failure (CHF). Symptoms of cardiac asthma are related to the heart's inability to effectively and efficiently pump blood in a CHF patient. This can lead to accumulation of fluid in and around the lungs, disrupting the lung's ability to oxygenate blood.

Cribbing is a form of stereotypy, otherwise known as wind sucking or crib-biting. Cribbing is considered to be an abnormal, compulsive behavior seen in some horses, and is often labelled a stable vice. The major factors that cause cribbing include stress, stable management, genetic and gastrointestinal irritability.
Robert Cook is an equine veterinarian. He has published many papers, mainly on diseases of the horse's mouth, ear, nose and throat both in scientific and horseman's journals, covering various topics:
Recurrent airway obstruction, also known as broken wind, heaves, wind-broke horse, or sometimes by the term usually reserved for humans, chronic obstructive pulmonary disease or disorder (COPD) – it is a respiratory disease or chronic condition of horses involving an allergic bronchitis characterised by wheezing, coughing and laboured breathing.
Equine polysaccharide storage myopathy is a hereditary glycogen storage disease of horses that causes exertional rhabdomyolysis. It is currently known to affect the following breeds American Quarter Horses, American Paint Horses, Warmbloods, Cobs, Dales Ponies, Thoroughbreds, Arabians, New Forest ponies, and a large number of Heavy horse breeds. While incurable, PSSM can be managed with appropriate diet and exercise. There are currently 2 subtypes, known as Type 1 PSSM and Type 2 PSSM.

A tongue-tie is a piece of equipment used by equestrians to prevent a horse from getting its tongue over the bit, which would make the animal very difficult to control. It is usually a strip of cloth or rubber, passed through the mouth and tied below the chin.

The circulatory system of the horse consists of the heart, the blood vessels, and the blood.

The respiratory system of the horse is the biological system by which a horse circulates air for the purpose of gaseous exchange.
Colitis X, equine colitis X or peracute toxemic colitis is a catchall term for various fatal forms of acute or peracute colitis found in horses, but particularly a fulminant colitis where clinical signs include sudden onset of severe diarrhea, abdominal pain, shock, and dehydration. Death is common, with 90–100% mortality, usually in less than 24 hours. The causative factor may be Clostridioides difficile, but it also may be caused by other intestinal pathogens. Horses under stress appear to be more susceptible to developing colitis X, and like the condition pseudomembranous colitis in humans, an association with prior antibiotic use also exists. Immediate and aggressive treatment can sometimes save the horse, but even in such cases, 75% mortality is considered a best-case scenario.

Guttural pouches are large, auditory-tube diverticula that contain between 300 and 600 ml of air. They are present in odd-toed mammals, some bats, hyraxes, and the American forest mouse. They are paired bilaterally just below the ears, behind the skull and connect to the nasopharynx.
Racehorse injuries and fatalities are a side effect of the training and competition of horse racing. Racehorse injuries are considered especially difficult to treat, as they frequently result in the death of the horse. A 2005 study by the United States Department of Agriculture found that injuries are the second leading cause of death in horses, second only to old age.

A nasal strip, external nasal dilator strip or nasal dilator strip is a type of adhesive bandage with embedded plastic ribs or splints that is applied across the bridge of the nose and sides of the nostrils, to assist in keeping the airway open. They are believed to make breathing easier and for that reason are used during athletic exertion and as an aid to reduce congestion or to prevent snoring. Various studies have indicated that they do not have a performance-enhancing effect. They are also used by race horse trainers on horses for similar reasons; they are thought to reduce airway resistance and lower the risk of exercise-induced pulmonary hemorrhage (EIPH), plus reduce fatigue and aid post-race recovery.
Equine gastric ulcer syndrome (EGUS) is a common cause of colic and decreased performance in horses. Horses form ulcers in the mucosa of the stomach, leading to pain, decreased appetite, weight loss, and behavioral changes. Treatment generally involves reducing acid production of the stomach and dietary management. Unlike some animals, however, stomach rupture is rare, and the main goal of treating is to reduce pain and improve performance of animals used for showing or racing.
Susan Marie Stover is a professor of veterinary anatomy at the University of California, Davis School of Veterinary Medicine and director of the J.D. Wheat Veterinary Orthopedic Research Laboratory. One of the focuses of her wide-ranging research is musculoskeletal injuries in racehorses, particularly catastrophic breakdowns. Her identification of risk factors has resulted in improved early detection and changes to horse training and surgical repair methods. On July 30, 2016, Stover received the Lifetime Excellence in Research Award from the American Veterinary Medical Association. In August 2016, she was selected for induction into the University of Kentucky Equine Research Hall of Fame.

The horse tongue, similar to that of most mammals, is pink and plays a significant role in taste perception. Its long, narrow shape, characteristic of herbivorous animals, allows the horse to grasp food effectively with the assistance of its lips and teeth. The tongue is sensitive to pressure and temperature and is involved in activities such as licking and chewing. While a mare licks her foal extensively immediately after birth, there is limited research on the gustatory sensitivity of horses and the social functions of their tongues.










