Related Research Articles

In chemistry, Ferric refers to the element iron in its +3 oxidation state. Ferric chloride is an alternative name for iron(III) chloride (FeCl3). The adjective ferrous is used instead for iron(II) salts, containing the cation Fe2+. The word ferric is derived from the Latin word ferrum, meaning "iron".

In chemistry, iron(II) refers to the element iron in its +2 oxidation state. The adjective ferrous or the prefix ferro- is often used to specify such compounds, as in ferrous chloride for iron(II) chloride (FeCl2). The adjective ferric is used instead for iron(III) salts, containing the cation Fe3+. The word ferrous is derived from the Latin word ferrum, meaning "iron".
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate ligand and a single central metal atom. These ligands are called chelants, chelators, chelating agents, or sequestering agents. They are usually organic compounds, but this is not a necessity.
Siderophores (Greek: "iron carrier") are small, high-affinity iron-chelating compounds that are secreted by microorganisms such as bacteria and fungi. They help the organism accumulate iron. Although a widening range of siderophore functions is now being appreciated, siderophores are among the strongest (highest affinity) Fe3+ binding agents known. Phytosiderophores are siderophores produced by plants.
The Suzuki reaction or Suzuki coupling is an organic reaction that uses a palladium complex catalyst to cross-couple a boronic acid to an organohalide. It was first published in 1979 by Akira Suzuki, and he shared the 2010 Nobel Prize in Chemistry with Richard F. Heck and Ei-ichi Negishi for their contribution to the discovery and development of noble metal catalysis in organic synthesis. This reaction is sometimes telescoped with the related Miyaura borylation; the combination is the Suzuki–Miyaura reaction. It is widely used to synthesize polyolefins, styrenes, and substituted biphenyls.

A quintuple bond in chemistry is an unusual type of chemical bond, first reported in 2005 for a dichromium compound. Single bonds, double bonds, and triple bonds are commonplace in chemistry. Quadruple bonds are rarer and are currently known only among the transition metals, especially for Cr, Mo, W, and Re, e.g. [Mo2Cl8]4− and [Re2Cl8]2−. In a quintuple bond, ten electrons participate in bonding between the two metal centers, allocated as σ2π4δ4.

In organic chemistry, phosphonates or phosphonic acids are organophosphorus compounds containing C−PO(OR)2 groups. Phosphonic acids, typically handled as salts, are generally nonvolatile solids that are poorly soluble in organic solvents, but soluble in water and common alcohols.

In organic chemistry, hydroxamic acids are a class of organic compounds having a general formula R−C(=O)−N(−OH)−R' bearing the functional group −C(=O)−N(−OH)−, where R and R' are typically organyl groups or hydrogen. They are amides wherein the nitrogen atom has a hydroxyl substituent. They are often used as metal chelators.
Metalloprotease inhibitors are cellular inhibitors of the Matrix metalloproteinases (MMPs). MMPs belong to a family of zinc-dependent neutral endopeptidases. These enzymes have the ability to break down connective tissue. The expression of MMPs is increased in various pathological conditions like inflammatory conditions, metabolic bone disease, to cancer invasion, metastasis and angiogenesis. Examples of diseases are periodontitis, hepatitis, glomerulonephritis, atherosclerosis, emphysema, asthma, autoimmune disorders of skin and dermal photoaging, rheumatoid arthritis, osteoarthritis, multiple sclerosis, Alzheimer's disease, chronic ulcerations, uterine involution, corneal epithelial defects, bone resorption and tumor progression and metastasis. Due to the role of MMPs in pathological conditions, inhibitors of MMPs may have therapeutic potential. Several other proteins have similar inhibitory effects, however none as effective. They might have other biological activities which have yet been fully characterised.
2,3-Dihydroxybenzoic acid is a natural phenol found in Phyllanthus acidus and in the aquatic fern Salvinia molesta. It is also abundant in the fruits of Flacourtia inermis. It is a dihydroxybenzoic acid, a type of organic compound.
Asymmetric hydrogenation is a chemical reaction that adds two atoms of hydrogen to a target (substrate) molecule with three-dimensional spatial selectivity. Critically, this selectivity does not come from the target molecule itself, but from other reagents or catalysts present in the reaction. This allows spatial information to transfer from one molecule to the target, forming the product as a single enantiomer. The chiral information is most commonly contained in a catalyst and, in this case, the information in a single molecule of catalyst may be transferred to many substrate molecules, amplifying the amount of chiral information present. Similar processes occur in nature, where a chiral molecule like an enzyme can catalyse the introduction of a chiral centre to give a product as a single enantiomer, such as amino acids, that a cell needs to function. By imitating this process, chemists can generate many novel synthetic molecules that interact with biological systems in specific ways, leading to new pharmaceutical agents and agrochemicals. The importance of asymmetric hydrogenation in both academia and industry contributed to two of its pioneers — William Standish Knowles and Ryōji Noyori — being collectively awarded one half of the 2001 Nobel Prize in Chemistry.

In coordination chemistry, denticity refers to the number of donor groups in a given ligand that bind to the central metal atom in a coordination complex. In many cases, only one atom in the ligand binds to the metal, so the denticity equals one, and the ligand is said to be monodentate. Ligands with more than one bonded atom are called polydentate or multidentate. The denticity of a ligand is described with the Greek letter κ ('kappa'). For example, κ6-EDTA describes an EDTA ligand that coordinates through 6 non-contiguous atoms.
In chemistry, hyponitrite may refer to the anion N
2O2−
2 ([ON=NO]2−), or to any ionic compound that contains it. In organic chemistry, it may also refer to the group −O−N=N−O−, or any organic compound with the generic formula R1−O−N=N−O−R2, where R1 and R2 are organic groups. Such compounds can be viewed as salts and esters of hyponitrous acid. An acid hyponitrite is an ionic compound with the anion HN
2O−
2 ([HON=NO]−).

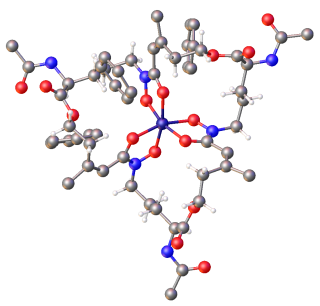
Ferrichrome is a cyclic hexa-peptide that forms a complex with iron atoms. It is a siderophore composed of three glycine and three modified ornithine residues with hydroxamate groups [-N(OH)C(=O)C-]. The 6 oxygen atoms from the three hydroxamate groups bind Fe(III) in near perfect octahedral coordination.
Many bacteria secrete small iron-binding molecules called siderophores, which bind strongly to ferric ions. FepA is an integral bacterial outer membrane porin protein that belongs to outer membrane receptor family and provides the active transport of iron bound by the siderophore enterobactin from the extracellular space, into the periplasm of Gram-negative bacteria. FepA has also been shown to transport vitamin B12, and colicins B and D as well. This protein belongs to family of ligand-gated protein channels.
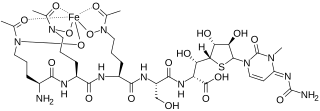
Albomycins are a group of naturally occurring antibiotics belonging to the class of sideromycins, which are "compounds composed of iron carriers called siderophores linked to antibiotic moieties". They are particularly effective against Gram-negative bacteria of the family Enterobacteriaceae and few Gram-positive bacteria such s Streptococcus pneumoniae, Bacillus subtilis and Staphylococcus aureus. In 2000 a group of scientists from SmithKline Beecham Pharmaceuticals, UK reported that the antibiotic part of albomycin in vitro can inhibit seryl-tRNA synthetase from both eukaryotic and prokaryotic representatives.
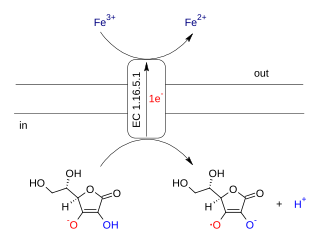
Ascorbate ferrireductase (transmembrane) (EC 1.16.5.1, cytochrome b561) is an enzyme with systematic name Fe(III):ascorbate oxidorectuctase (electron-translocating). This enzyme catalyses the following chemical reaction
Siderocalin(Scn), lipocalin-2, NGAL, 24p3 is a mammalian lipocalin-type protein that can prevent iron acquisition by pathogenic bacteria by binding siderophores, which are iron-binding chelators made by microorganisms. Iron serves as a key nutrient in host-pathogen interactions, and pathogens can acquire iron from the host organism via synthesis and release siderophores such as enterobactin. Siderocalin is a part of the mammalian defence mechanism and acts as an antibacterial agent. Crystallographic studies of Scn demonstrated that it includes a calyx, a ligand-binding domain that is lined with polar cationic groups. Central to the siderophore/siderocalin recognition mechanism are hybrid electrostatic/cation-pi interactions. To evade the host defences, pathogens evolved to produce structurally varied siderophores that would not be recognized by siderocalin, allowing the bacteria to acquire iron.

Rebecca Abergel is a French inorganic chemist who specializes in the coordination chemistry between lanthanide and actinide complexes. Alongside the effects of heavy element exposure and contamination on different biological systems. Abergel is currently a faculty scientist and heavy element chemistry group leader at the chemical sciences division of Lawrence Berkeley National Laboratory in Berkeley, California. She is also assistant professor of nuclear engineering at University of California, Berkeley.
References
- ↑ Candeloro, Sofia; Grdenić, D.; Taylor, Noel; Thompson, B.; Viswamitra, M.; Hodgkin, Dorothy Crowfoot (1969). "Structure of ferroverdin". Nature . 224 (5219): 589–591. Bibcode:1969Natur.224..589C. doi:10.1038/224589a0. PMID 5346597. S2CID 4288012.
- 1 2 3 4 5 Neilands, J. B. (1966). "Naturally occurring non-porphyrin iron compounds". Structure and Bonding. Vol. 1. pp. 59–108. doi:10.1007/BFb0119549. ISBN 978-3-540-03675-3.
- ↑ Cone, M (1995). "4-Hydroxy-3-nitrosobenzamide and its ferrous chelate from Streptomyces murayamaensis". Tetrahedron. 51 (11): 3095–3102. doi:10.1016/0040-4020(95)00071-F.
- 1 2 3 Tomoda, H; Tabata, N; Shinose, M; Takahashi, Y & Woodruff, H. (1999). "Ferroverdins, inhibitors of cholesteryl ester transfer protein produced by streptomyces sp. wk-5344. i. production, isolation and biological properties". The Journal of Antibiotics . 52 (12): 1101–1107. doi: 10.7164/antibiotics.52.1101 . PMID 10695673.
- ↑ Surajit Chattopadhyay; Partha Basu; Debashis Ray; Samudranil Pal; Animesh Chakravorty (1990). "Ferroverdin: Cation variation and recognition of isomerictris chelate geometries by iron oxidation states". Journal of Chemical Sciences. 102 (3): 195–202. doi: 10.1007/BF02841933 . S2CID 92660666.