Olive oil is a liquid fat obtained by pressing whole olives, the fruit of Olea europaea, a traditional tree crop of the Mediterranean Basin, and extracting the oil.

Fennel is a flowering plant species in the carrot family. It is a hardy, perennial herb with yellow flowers and feathery leaves. It is indigenous to the shores of the Mediterranean but has become widely naturalized in many parts of the world, especially on dry soils near the sea coast and on riverbanks.

Linalool refers to two enantiomers of a naturally occurring terpene alcohol found in many flowers and spice plants. Linalool has multiple commercial applications, the majority of which are based on its pleasant scent. A colorless oil, linalool is classified as an acyclic monoterpenoid. In plants, it is a metabolite, a volatile oil component, an antimicrobial agent, and an aroma compound. Linalool has uses in manufacturing of soaps, fragrances, food additives as flavors, household products, and insecticides. Esters of linalool are referred to as linalyl, e.g. linalyl pyrophosphate, an isomer of geranyl pyrophosphate.

Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric products in unequal amounts."
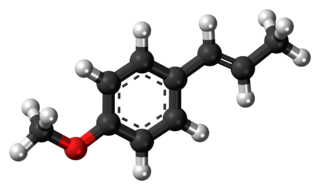
Anethole is an organic compound that is widely used as a flavoring substance. It is a derivative of the aromatic compound allylbenzene and occurs widely in the essential oils of plants. It is in the class of phenylpropanoid organic compounds. It contributes a large component of the odor and flavor of anise and fennel, anise myrtle (Myrtaceae), liquorice (Fabaceae), magnolia blossoms, and star anise (Schisandraceae). Closely related to anethole is its isomer estragole, which is abundant in tarragon (Asteraceae) and basil (Lamiaceae), and has a flavor reminiscent of anise. It is a colorless, fragrant, mildly volatile liquid. Anethole is only slightly soluble in water but exhibits high solubility in ethanol. This trait causes certain anise-flavored liqueurs to become opaque when diluted with water; this is called the ouzo effect.

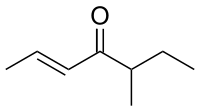
Carvone is a member of a family of chemicals called terpenoids. Carvone is found naturally in many essential oils, but is most abundant in the oils from seeds of caraway, spearmint, and dill.
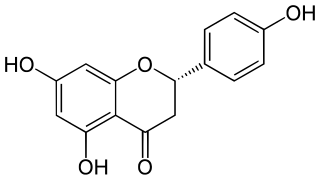
Naringenin is a flavanone from the flavonoid group of polyphenols. It is commonly found in citrus fruits, especially as the predominant flavonone in grapefruit.
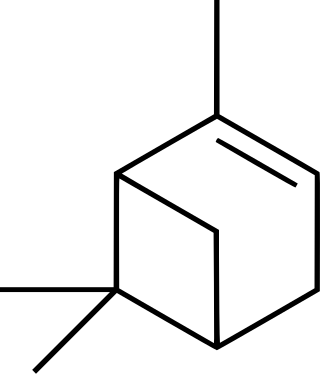
Pinene is a collection of unsaturated bicyclic monoterpenes. Two geometric isomers of pinene are found in nature, α-pinene and β-pinene. Both are chiral. As the name suggests, pinenes are found in pines. Specifically, pinene is the major component of the liquid extracts of conifers. Pinenes are also found in many non-coniferous plants such as camphorweed (Heterotheca) and big sagebrush.
Chiral column chromatography is a variant of column chromatography that is employed for the separation of chiral compounds, i.e. enantiomers, in mixtures such as racemates or related compounds. The chiral stationary phase (CSP) is made of a support, usually silica based, on which a chiral reagent or a macromolecule with numerous chiral centers is bonded or immobilized.

Eucalyptol is a monoterpenoid colorless liquid, and a bicyclic ether. It has a fresh camphor-like odor and a spicy, cooling taste. It is insoluble in water, but miscible with organic solvents. Eucalyptol makes up about 70–90% of eucalyptus oil. Eucalyptol forms crystalline adducts with hydrohalic acids, o-cresol, resorcinol, and phosphoric acid. Formation of these adducts is useful for purification.

Myrcene, or β-myrcene, is a monoterpene. A colorless oil, it occurs widely in essential oils. It is produced mainly semi-synthetically from Myrcia, from which it gets its name. It is an intermediate in the production of several fragrances. α-Myrcene is the name for the isomer 2-methyl-6-methylene-1,7-octadiene, which has not been found in nature.

In analytical chemistry, a chiral derivatizing agent (CDA), also known as a chiral resolving reagent, is a derivatization reagent that is a chiral auxiliary used to convert a mixture of enantiomers into diastereomers in order to analyze the quantities of each enantiomer present and determine the optical purity of a sample. Analysis can be conducted by spectroscopy or by chromatography. Some analytical techniques such as HPLC and NMR, in their most commons forms, cannot distinguish enantiomers within a sample, but can distinguish diastereomers. Therefore, converting a mixture of enantiomers to a corresponding mixture of diastereomers can allow analysis. The use of chiral derivatizing agents has declined with the popularization of chiral HPLC. Besides analysis, chiral derivatization is also used for chiral resolution, the actual physical separation of the enantiomers.

Avocado oil is an edible oil extracted from the pulp of avocados, the fruit of Persea americana. It is used as an edible oil both raw and for cooking, where it is noted for its high smoke point. It is also used for lubrication and in cosmetics.

Fenchone is an organic compound classified as a monoterpenoid and a ketone. It is a colorless oily liquid. It has a structure and an odor similar to those of camphor. Fenchone is a constituent of absinthe and the essential oil of fennel. Fenchone is used as a flavor in foods and in perfumery.

Olive leaf is the leaf of the olive tree. Although olive oil is well known for its flavor and possible health benefits, the leaf and its extracts remain under preliminary research with unknown effects on human health.
Amar en tiempos revueltos, is a Spanish television period soap opera that originally ran on La 1 of Televisión Española for seven seasons, from 27 September 2005 to 16 November 2012, set in the times of the Spanish civil war and Francoist Spain.

Olive oil regulation and adulteration are complex issues overseen and studied by various governmental bodies, non-governmental organizations, and private researchers across the world. The most frequent type of adulteration is that oil of lower quality is mixed into olive oil.
2-Methylundecanal is an organic compound that is found naturally in kumquat peel oil. This compound smells herbaceous, orange, and ambergris-like. At high dilution it has a flavor similar to honey and nuts. It is a colorless or pale yellow liquid that is soluble in organic solvents such as ether and ethanol. It is used as a fragrance component in soaps, detergents, and perfumes.

Bergamot essential oil is a cold-pressed essential oil produced by cells inside the rind of a bergamot orange fruit. It is a common flavoring and top note in perfumes. The scent of bergamot essential oil is similar to a sweet light orange peel oil with a floral note.
Chiral analysis refers to the quantification of component enantiomers of racemic drug substances or pharmaceutical compounds. Other synonyms commonly used include enantiomer analysis, enantiomeric analysis, and enantioselective analysis. Chiral analysis includes all analytical procedures focused on the characterization of the properties of chiral drugs. Chiral analysis is usually performed with chiral separation methods where the enantiomers are separated on an analytical scale and simultaneously assayed for each enantiomer.