
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene, and has numerous applications because it is chemically inert. The commonly known brand name of PTFE-based composition is Teflon by Chemours, a spin-off from DuPont, which originally invented the compound in 1938. Polytetrafluoroethylene is a fluorocarbon solid, as it is a high-molecular-weight polymer consisting wholly of carbon and fluorine. PTFE is hydrophobic: neither water nor water-containing substances wet PTFE, as fluorocarbons exhibit only small London dispersion forces due to the low electric polarizability of fluorine. PTFE has one of the lowest coefficients of friction of any solid.
Roy Joseph Plunkett was an American chemist. He discovered polytetrafluoroethylene (PTFE), better known as Teflon, in 1938.

Gore-Tex is W. L. Gore & Associates's trade name for waterproof, breathable fabric membrane. It was invented in 1969. Gore-Tex blocks liquid water while allowing water vapor to pass through and is designed to be a lightweight, waterproof fabric for all-weather use. It is composed of expanded PTFE (ePTFE), a stretched out form of the PFAS compound polytetrafluoroethylene (PTFE).
A fluoropolymer is a fluorocarbon-based polymer with multiple carbon–fluorine bonds. It is characterized by a high resistance to solvents, acids, and bases. The best known fluoropolymer is polytetrafluoroethylene under the brand name "Teflon," trademarked by the DuPont Company.

Ethylene tetrafluoroethylene (ETFE) is a fluorine-based plastic. It was designed to have high corrosion resistance and strength over a wide temperature range. ETFE is a polymer and its source-based name is poly (ethene-co-tetrafluoroethene). It is also known under the DuPont brand name Tefzel and is sometimes referred to as 'Teflon Film'. ETFE has a relatively high melting temperature and excellent chemical, electrical and high-energy radiation resistance properties.
Tetrafluoroethylene (TFE) is a fluorocarbon with the chemical formula C2F4. It is the simplest perfluorinated alkene. This gaseous species is used primarily in the industrial preparation of fluoropolymers.

ECTFE (ethylene-chlorotrifluoroethylene) is an alternating copolymer of ethylene and chlorotrifluoroethylene. It is a semi-crystalline fluoropolymer, with chemical corrosion resistance properties.
FKM is a family of fluorocarbon-based fluoroelastomer materials defined by ASTM International standard D1418, and ISO standard 1629. It is commonly called fluorine rubber or fluoro-rubber. FKM is an abbreviation of Fluorine Kautschuk Material. All FKMs contain vinylidene fluoride as the common monomer, to which different other monomers are added for specific types and functionalities, fitting the desired application.
Teflon is a registered trademark of the Chemours company used for polytetrafluoroethylene.
Perfluoroethers are a class of organofluorine compound containing one or more ether functional group. In general these compounds are structurally analogous to the related hydrocarbon ethers, except for the distinctive properties of fluorocarbons.

A 'non-stick surface' is engineered to reduce the ability of other materials to stick to it. Non-sticking cookware is a common application, where the non-stick coating allows food to brown without sticking to the pan. Non-stick is often used to refer to surfaces coated with polytetrafluoroethylene (PTFE), a well-known brand of which is Teflon. In the twenty-first century, other coatings have been marketed as non-stick, such as anodized aluminium, silica, enameled cast iron, and seasoned cookware.
Electron-beam processing or electron irradiation (EBI) is a process that involves using electrons, usually of high energy, to treat an object for a variety of purposes. This may take place under elevated temperatures and nitrogen atmosphere. Possible uses for electron irradiation include sterilization, alteration of gemstone colors, and cross-linking of polymers.

Hexafluoropropylene oxide (HFPO) is an intermediate used in industrial organofluorine chemistry; specifically it is a monomer for fluoropolymers. This colourless gas is the epoxide of hexafluoropropylene, which is a fluorinated analog of propylene oxide, HFPO is produced by Chemours and 3M and as a precursor to the lubricant Krytox and related materials. It is generated by oxidation of perfluoropropylene, e.g. with oxygen as well as other oxidants.
A perfluorinated compound (PFC) or perfluoro compound is an organofluorine compound that lacks C-H bonds. Many perfluorinated compounds have properties that are quite different from their C-H containing analogues. Common functional groups in PFCs are OH, CO2H, chlorine, O, and SO3H. Electrofluorination is the predominant method for PFC production. Due to their chemical stability, some of these perfluorinated compounds bioaccumulate.
Xylan is a fluoropolymer-based industrial coating, most commonly used in non-stick cookware. Generally, it is applied in a thin film to the target material to improve its durability and non-stick properties.
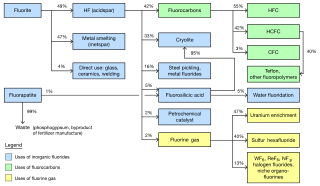
The global market for chemicals from fluorine was about US$16 billion per year as of 2006. The industry was predicted to reach 2.6 million metric tons per year by 2015. The largest market is the United States. Western Europe is the second largest. Asia Pacific is the fastest growing region of production. China in particular has experienced significant growth as a fluorochemical market and is becoming a producer of them as well. Fluorite mining was estimated in 2003 to be a $550 million industry, extracting 4.5 million tons per year.

Perfluoroalkoxy alkanes (PFA) are fluoropolymers. They are copolymers of tetrafluoroethylene (C2F4) and perfluoroethers (C2F3ORf, where Rf is a perfluorinated group such as trifluoromethyl (CF3)). The properties of these polymers are similar to those of polytetrafluoroethylene (PTFE). Compared to PTFE, PFA has better anti-stick properties and higher chemical resistance, at the expense of lesser scratch resistance.
The Chemours Company is an American chemical company that was founded in July 2015 as a spin-off from DuPont. It has its corporate headquarters in Wilmington, Delaware, United States. Chemours is the manufacturer of Teflon, the brand name of polytetrafluoroethylene (PTFE), known for its anti-stick properties. It also produces titanium dioxide and refrigerant gases. It is currently being sued by the PA Attorney General, for knowingly exposing the public to PFAS.

Herbert S. Eleuterio was an American industrial chemist noted for technical contributions to catalysis, polymerization, industrial research management, and science education. In particular, he discovered the olefin metathesis reaction and several novel fluoropolymers. Additionally, he explored techniques for research leadership, especially methods for fostering collaboration, globalization, and scientific creativity.










