
Gore-Tex is W. L. Gore & Associates's trade name for waterproof, breathable fabric membrane. It was invented in 1969. Gore-Tex blocks liquid water while allowing water vapor to pass through and is designed to be a lightweight, waterproof fabric for all-weather use. It is composed of expanded PTFE (ePTFE), a stretched out form of the PFAS compound polytetrafluoroethylene (PTFE).

Polyvinylidene fluoride or polyvinylidene difluoride (PVDF) is a highly non-reactive thermoplastic fluoropolymer produced by the polymerization of vinylidene difluoride. Its chemical formula is (C2H2F2)n.

Perfluorooctanoic acid is a perfluorinated carboxylic acid produced and used worldwide as an industrial surfactant in chemical processes and as a material feedstock. PFOA is considered a surfactant, or fluorosurfactant, due to its chemical structure, which consists of a perfluorinated, n-heptyl "tail group" and a carboxylic acid "head group". The head group can be described as hydrophilic while the fluorocarbon tail is both hydrophobic and lipophobic.
A fluoropolymer is a fluorocarbon-based polymer with multiple carbon–fluorine bonds. It is characterized by a high resistance to solvents, acids, and bases. The best known fluoropolymer is polytetrafluoroethylene under the brand name "Teflon," trademarked by the DuPont Company.
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is R−:C−R' or R=C: where the R represents substituents or hydrogen atoms.
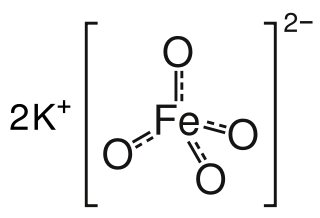
Potassium ferrate is an inorganic compound with the formula K2FeO4. It is the potassium salt of ferric acid. Potassium ferrate is a powerful oxidizing agent with applications in green chemistry, organic synthesis, and cathode technology.

Nonylphenols are a family of closely related organic compounds composed of phenol bearing a 9 carbon-tail. Nonylphenols can come in numerous structures, all of which may be considered alkylphenols. They are used in manufacturing antioxidants, lubricating oil additives, laundry and dish detergents, emulsifiers, and solubilizers. They are used extensively in epoxy formulation in North America but its use has been phased out in Europe. These compounds are also precursors to the commercially important non-ionic surfactants alkylphenol ethoxylates and nonylphenol ethoxylates, which are used in detergents, paints, pesticides, personal care products, and plastics. Nonylphenol has attracted attention due to its prevalence in the environment and its potential role as an endocrine disruptor and xenoestrogen, due to its ability to act with estrogen-like activity. The estrogenicity and biodegradation heavily depends on the branching of the nonyl sidechain. Nonylphenol has been found to act as an agonist of the GPER (GPR30).
Tetrafluoroethylene (TFE) is a fluorocarbon with the chemical formula C2F4. It is the simplest perfluorinated alkene. This gaseous species is used primarily in the industrial preparation of fluoropolymers.
Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain a carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents.

Sulfolene, or butadiene sulfone is a cyclic organic chemical with a sulfone functional group. It is a white, odorless, crystalline, indefinitely storable solid, which dissolves in water and many organic solvents. The compound is used as a source of butadiene.
Perfluoroethers are a class of organofluorine compound containing one or more ether functional group. In general these compounds are structurally analogous to the related hydrocarbon ethers, except for the distinctive properties of fluorocarbons.

Hexafluoropropylene oxide (HFPO) is an intermediate used in industrial organofluorine chemistry; specifically it is a monomer for fluoropolymers. This colourless gas is the epoxide of hexafluoropropylene, which is a fluorinated analog of propylene oxide, HFPO is produced by Chemours and 3M and as a precursor to the lubricant Krytox and related materials. It is generated by oxidation of perfluoropropylene, e.g. with oxygen as well as other oxidants.

Fluorine perchlorate, also called perchloryl hypofluorite is the rarely encountered chemical compound of fluorine, chlorine, and oxygen with the chemical formula ClO
4F or FOClO
3. It is an extremely unstable gas that explodes spontaneously and has a penetrating odor.
Telomerization is a reaction that produces a particular kind of oligomer with two distinct end groups. The oligomer is called a telomer. Some telomerizations proceed by radical pathways, many do not. A generic equation is:
Fluorotelomer alcohols, or FTOHs, are fluorotelomers with an alcohol functional group. They are volatile precursors to perfluorinated carboxylic acids, such as PFOA and PFNA, and other compounds.
Fluorotelomers are fluorocarbon-based oligomers, or telomers, synthesized by telomerization. Some fluorotelomers and fluorotelomer-based compounds are a source of environmentally persistent perfluorinated carboxylic acids such as PFOA and PFNA, while others are under extended investigation.
Surflon S-111 is a commercial product consisting of perfluorinated carboxylic acids (PFCAs) in ammonium salt form. It is commonly used as a polymerization aid in the production of fluoropolymers.
Difluorocarbene is the chemical compound with formula CF2. It has a short half-life, 0.5 and 20 ms, in solution and in the gas phase, respectively. Although highly reactive, difluorocarbene is an intermediate in the production of tetrafluoroethylene, which is produced on an industrial scale as the precursor to Teflon (PTFE).

Perfluorooctanesulfonyl fluoride (POSF) is a synthetic perfluorinated compound with a sulfonyl fluoride functional group. It is used to make perfluorooctanesulfonic acid (PFOS) and PFOS-based compounds. These compounds have a variety of industrial and consumer uses, but POSF-derived substances ultimately degrade to form PFOS.
Perfluoropolyethers (PFPEs) are a class of organofluorine compound. Some types are synthetic liquid lubricants that have been used in the aerospace industry for over 30 years. The main properties of PFPE are being temperature resistant between −58 °C (215 K) and 257 °C (530 K), having very low outgassing compared to other fluids and having a dielectric strength of around 15.7 MV/m.














