
A phospholipase is an enzyme that hydrolyzes phospholipids into fatty acids and other lipophilic substances. There are four major classes, termed A, B, C, and D, which are distinguished by the type of reaction which they catalyze:

The enzyme phospholipase A2 (EC 3.1.1.4, PLA2, systematic name phosphatidylcholine 2-acylhydrolase) catalyses the cleavage of fatty acids in position 2 of phospholipids, hydrolyzing the bond between the second fatty acid "tail" and the glycerol molecule:
Taicatoxin (TCX) is a snake toxin that blocks voltage-dependent L-type calcium channels and small conductance Ca2+-activated K+ channels. The name taicatoxin (TAIpan + CAlcium + TOXIN) is derived from its natural source, the taipan snake, the site of its action, calcium channels, and from its function as a toxin. Taicatoxin was isolated from the venom of Australian taipan snake, Oxyuranus scutellatus scutellatus. TCX is a secreted protein, produced in the venom gland of the snake.

Phospholipase A1 (EC 3.1.1.32; systematic name: phosphatidylcholine 1-acylhydrolase) encoded by the PLA1A gene is a phospholipase enzyme which removes the 1-acyl group:

Methoxy arachidonyl fluorophosphonate, commonly referred as MAFP, is an irreversible active site-directed enzyme inhibitor that inhibits nearly all serine hydrolases and serine proteases. It inhibits phospholipase A2 and fatty acid amide hydrolase with special potency, displaying IC50 values in the low-nanomolar range. In addition, it binds to the CB1 receptor in rat brain membrane preparations (IC50 = 20 nM), but does not appear to agonize or antagonize the receptor, though some related derivatives do show cannabinoid-like properties.

Phospholipase A2, membrane associated is an enzyme that in humans is encoded by the PLA2G2A gene.

Phospholipase C (PLC) is a class of membrane-associated enzymes that cleave phospholipids just before the phosphate group (see figure). It is most commonly taken to be synonymous with the human forms of this enzyme, which play an important role in eukaryotic cell physiology, in particular signal transduction pathways. Phospholipase C's role in signal transduction is its cleavage of phosphatidylinositol 4,5-bisphosphate (PIP2) into diacyl glycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), which serve as second messengers. Activators of each PLC vary, but typically include heterotrimeric G protein subunits, protein tyrosine kinases, small G proteins, Ca2+, and phospholipids.

Annexin A4 is a protein that in humans is encoded by the ANXA4 gene.

Ramoplanin (INN) is a glycolipodepsipeptide antibiotic drug derived from strain ATCC 33076 of Actinoplanes. It is effective against Gram-positive bacteria.

Manoalide is a calcium channel blocker. It has antibiotic, analgesic and anti-inflammatory effects and is found in some sponges, including the West Pacific species Luffariella variabilis. Its functions are made possible by the permanent blockage of phospholipase A2 and C with lysine residues. This could be made possible through the functional groups incorporated in gamma-hydroxybutenolide, alpha-hydroxydihydropyran and the trimethylcyclohexenyl. The gamma-hydroxybutenolide ring is present in the reaction between manoalide and phospholipase A2, the hemiacetal in alpha-hydroxydihydropyran is needed for permanent binding and hydrophobic trimethylcyclohexenyl ring makes it possible for non-bonded interactions to interact between manoalide and phospholipase A2 to strengthen the reaction. Due to its potential of permanent inhibition, it was made possible for it to take part in oral cancer and hepatitis C research.

A depside is a type of polyphenolic compound composed of two or more monocyclic aromatic units linked by an ester group. Depsides are most often found in lichens, but have also been isolated from higher plants, including species of the Ericaceae, Lamiaceae, Papaveraceae and Myrtaceae.
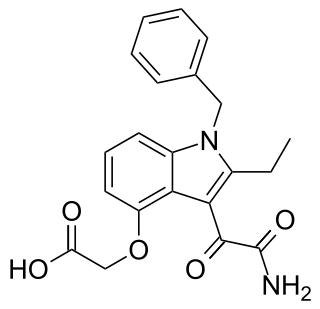
Varespladib is an inhibitor of the IIa, V, and X isoforms of secretory phospholipase A2 (sPLA2). The molecule acts as an anti-inflammatory agent by disrupting the first step of the arachidonic acid pathway of inflammation. From 2006 to 2012, varespladib was under active investigation by Anthera Pharmaceuticals as a potential therapy for several inflammatory diseases, including acute coronary syndrome and acute chest syndrome. The trial was halted in March 2012 due to inadequate efficacy. The selective sPLA2 inhibitor varespladib (IC50 value 0.009 μM in chromogenic assay, mole fraction 7.3X10-6) was studied in the VISTA-16 randomized clinical trial (clinicaltrials.gov Identifier: NCT01130246) and the results were published in 2014. The sPLA2 inhibition by varespladib in this setting seemed to be potentially harmful, and thus not a useful strategy for reducing adverse cardiovascular outcomes from acute coronary syndrome. Since 2016, scientific research has focused on the use of Varespladib as an inhibitor of snake venom toxins using various types of in vitro and in vivo models. Varespladib showed a significant inhibitory effect to snake venom PLA2 which makes it a potential first-line drug candidate in snakebite envenomation therapy. In 2019, the U.S. Food and Drug Administration (FDA) granted varespladib orphan drug status for its potential to treat snakebite.

Asterric acid is a fungal metabolite that can inhibit endothelin binding, first isolated from Aspergillus terreus. Its derivatives and similar phenolic fungal isolates are a subject of research on anti-angiogenic compounds.
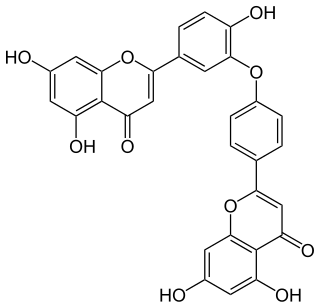
Ochnaflavone, a secondary plant secondary metabolite of the Biflavonoid family, has been widely investigated in past decades due to its unique ability to mediate biological activities, such as inhibition of phospholipase A2 and lymphocyte proliferation. It was first isolated from Ochna squarrosa Linn, a member of Ochnaceae family, in 1973.
Medicinal fungi are fungi that contain metabolites or can be induced to produce metabolites through biotechnology to develop prescription drugs. Compounds successfully developed into drugs or under research include antibiotics, anti-cancer drugs, cholesterol and ergosterol synthesis inhibitors, psychotropic drugs, immunosuppressants and fungicides.
Penicillium chermesinum is an anamorph fungus species of the genus of Penicillium which was isolated from soil from Nova Scotia in Canada.Penicillium chermesinum produces plastatin, luteosporin, xanthomegnin, azaphilones, p-terphenyls and costaclavine.
Aspergillus unguis is a species of fungus in the genus Aspergillus, and the asexual state (anamorph) of Emericella unguis. Aspergillus unguis is a filamentous soil-borne fungus found on decomposing plant matter and other moist substrates including with building materials and household dust. Aspergillus unguis occurs mainly in tropical and subtropical soils but has also been isolated from various marine and aquatic habitats. The species was first isolated in 1935 by Weill and L. Gaudin. Historically, A. unguis was assigned to the A. nidulans group, a common group of soil-borne fungi due to the resemblance of its ascospores and cleistothecia to those of Emericella nidulans. Aspergillus unguis is distinctive, however, in possessing spicular hyphae. A number of synonyms have been collapsed into this species, including Sterigmatocystis unguis, Aspergillus laokiashanensis and Aspergillus mellinus.
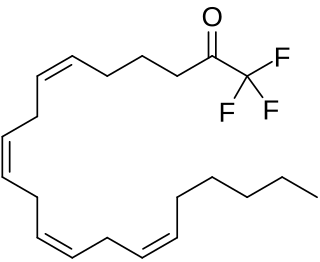
Arachidonyl trifluoromethyl ketone (ATK) is an analog of arachidonic acid. that inhibits some isoforms of the enzyme phospholipase A2. Specifically it inhibits the 85 kDa cystolic PLA2 (cPLA2).
Crotoxin (CTX) is the main toxic compound in the snake venom of the South American rattlesnake, Crotalus durissus terrificus. Crotoxin is a heterodimeric beta-neurotoxin, composed of an acidic, non-toxic and non-enzymatic subunit (CA), and a basic, weakly toxic, phospholipase A2 protein (CB). This neurotoxin causes paralysis by both pre- and postsynaptic blocking of acetylcholine signalling.
Wicklowia aquatica is a freshwater fungus species in the genus Wicklowia that is found in Florida and Costa Rica. Wicklowia aquatica produces the depsidone compound folipastatin.












