In organic chemistry, a hemiacetal or a hemiketal has the general formula R1R2C(OH)OR, where R1, R2 is hydrogen or an organic substituent. They generally result from the addition of an alcohol to an aldehyde or a ketone, although the latter are sometimes called hemiketals. Most sugars are hemiacetals.

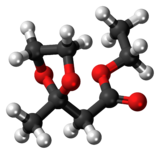
In organic chemistry, an acetal is a functional group with the connectivity R2C(OR')2. Here, the R groups can be organic fragments or hydrogen, while the R' groups must be organic fragments not hydrogen. The two R' groups can be equivalent to each other or not. Acetals are formed from and convertible to aldehydes or ketones and have the same oxidation state at the central carbon, but have substantially different chemical stability and reactivity as compared to the analogous carbonyl compounds. The central carbon atom has four bonds to it, and is therefore saturated and has tetrahedral geometry.
Perfume is a mixture of fragrant essential oils or aroma compounds (fragrances), fixatives and solvents, usually in liquid form, used to give the human body, animals, food, objects, and living-spaces an agreeable scent. Perfumes can be defined as substances that emit and diffuse a pleasant and fragrant odor. They consist of manmade mixtures of aromatic chemicals and essential oils. The 1939 Nobel Laureate for Chemistry, Leopold Ružička stated in 1945 that "right from the earliest days of scientific chemistry up to the present time, perfumes have substantially contributed to the development of organic chemistry as regards methods, systematic classification, and theory."

Ethylene glycol is an organic compound with the formula (CH2OH)2. It is mainly used for two purposes: as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odorless, colorless, flammable, viscous liquid. It has a sweet taste, but is toxic in high concentrations. This molecule has been observed in outer space.

An aroma compound, also known as an odorant, aroma, fragrance or flavoring, is a chemical compound that has a smell or odor. For an individual chemical or class of chemical compounds to impart a smell or fragrance, it must be sufficiently volatile for transmission via the air to the olfactory system in the upper part of the nose. As examples, various fragrant fruits have diverse aroma compounds, particularly strawberries which are commercially cultivated to have appealing aromas, and contain several hundred aroma compounds.
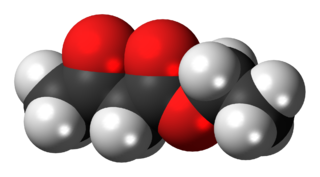
The organic compound ethyl acetoacetate (EAA) is the ethyl ester of acetoacetic acid. It is a colorless liquid. It is widely used as a chemical intermediate in the production of a wide variety of compounds.

Borneol is a bicyclic organic compound and a terpene derivative. The hydroxyl group in this compound is placed in an endo position. The exo diastereomer is called isoborneol. Being chiral, borneol exists as enantiomers, both of which are found in nature.
Dioxolane is a heterocyclic acetal with the chemical formula (CH2)2O2CH2. It is related to tetrahydrofuran (THF) by replacement of the methylene group (CH2) at the 2-position with an oxygen atom. The corresponding saturated 6-membered C4O2 rings are called dioxanes. The isomeric 1,2-dioxolane (wherein the two oxygen centers are adjacent) is a peroxide. 1,3-dioxolane is used as a solvent and as a comonomer in polyacetals.

The Danishefsky Taxol total synthesis in organic chemistry is an important third Taxol synthesis published by the group of Samuel Danishefsky in 1996 two years after the first two efforts described in the Holton Taxol total synthesis and the Nicolaou Taxol total synthesis. Combined they provide a good insight in the application of organic chemistry in total synthesis.

Bisabolol, or more formally α-(−)-bisabolol or also known as levomenol, is a natural monocyclic sesquiterpene alcohol. It is a colorless viscous oil that is the primary constituent of the essential oil from German chamomile and Myoporum crassifolium. High concentrations of bisabolol can also be found in certain medicinal cannabis cultivars. It is poorly soluble in water and glycerine, but soluble in ethanol. The enantiomer, α-(+)-bisabolol, is also found naturally but is rare. Synthetic bisabolol is usually a racemic mixture of the two, α-(±)-bisabolol. It is the terpenoid responsible for distinctive aroma of chamomile flowers, and when isolated, its scent has also has been likened to apples, sugar and honey.
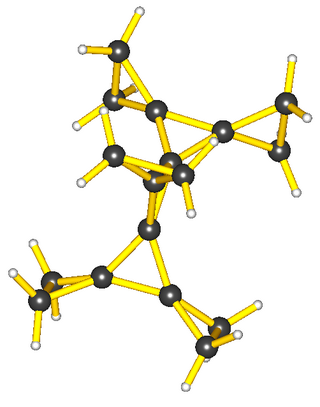
In organic chemistry, spiro compounds are compounds that have at least two molecular rings sharing one common atom. Simple spiro compounds are bicyclic. The presence of only one common atom connecting the two rings distinguishes spiro compounds from other bicyclics. Spiro compounds may be fully carbocyclic or heterocyclic. One common type of spiro compound encountered in educational settings is a heterocyclic one— the acetal formed by reaction of a diol with a cyclic ketone.

β-Pinene is a monoterpene, an organic compound found in plants. It is one of the two isomers of pinene, the other being α-pinene. It is a colorless liquid soluble in alcohol, but not water. It has a woody-green pine-like smell.

Eau de toilette is a lightly scented perfume. It is also referred to as aromatic waters and has a high alcohol content. It is usually applied directly to the skin after bathing or shaving. It is traditionally composed of alcohol and various volatile oils. Traditionally these products were named after a principal ingredient; some being geranium water, lavender water, lilac water, violet water, spirit of myrcia and 'eau de Bretfeld'. Because of this, eau de toilette was sometimes referred to as "toilet water".

Oxotrichlorobis(triphenylphosphine)rhenium(V) is the chemical compound with the formula ReOCl3(PPh3)2. This yellow, air-stable solid is a precursor to a variety of other rhenium complexes. In this diamagnetic compound, Re has an octahedral coordination environment with one oxo, three chloro and two mutually trans triphenylphosphine ligands. The oxidation state of rhenium is +5 and its configuration is d2.
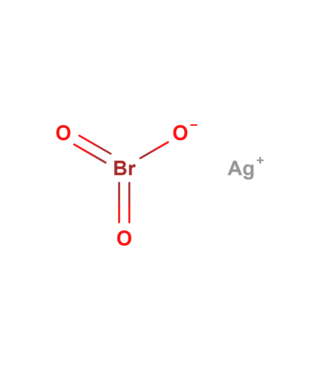
Silver bromate (AgBrO3), is a toxic, light and heat-sensitive, white powder.
Allyl hexanoate is an organic compound with the formula C5H11CO2CH2CH=CH2. It is a colorless liquid, although commercial samples appear yellowish. It occurs naturally in pineapples.
Celine Dion Parfums is a brand line of celebrity-endorsed perfumes by Canadian singer Celine Dion and Coty, with global retail sales of more than $850 million by March 2010. As of 2011, there have been 14 perfumes that have been released. A new fragrance called Signature, was released in September 2011.

Cyclopropenone is an organic compound with molecular formula C3H2O consisting of a cyclopropene carbon framework with a ketone functional group. It is a colorless, volatile liquid that boils near room temperature. Neat cyclopropenone polymerizes upon standing at room temperature, and chemical vendors typically supply it as an acetal. The chemical properties of the compound are dominated by the strong polarization of the carbonyl group, which gives a partial positive charge with aromatic stabilization on the ring and a partial negative charge on oxygen. It is an aromatic compound.

In organic chemistry, acetophenide is a functional group which is composed of the cyclic ketal of a diol with acetophenone. In pharmaceutical chemistry, it is present in algestone acetophenide and amcinafide.
In organic chemistry, aminobenzal is a functional group which is composed of a cyclic ketal of a diol with 4-dimethylaminobenzylidene. It is seen in triamcinolone aminobenzal benzamidoisobutyrate.