
Benzoic acid is a white (or colorless) solid with the formula C6H5CO2H. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, which was for a long time its only source. Benzoic acid occurs naturally in many plants and serves as an intermediate in the biosynthesis of many secondary metabolites. Salts of benzoic acid are used as food preservatives. Benzoic acid is an important precursor for the industrial synthesis of many other organic substances. The salts and esters of benzoic acid are known as benzoates.
The cumene process is an industrial process for synthesizing phenol and acetone from benzene and propylene. The term stems from cumene, the intermediate material during the process. It was invented by R. Ūdris and P. Sergeyev in 1942 (USSR)., and independently by Heinrich Hock in 1944

A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.

Benzyl alcohol is an aromatic alcohol with the formula C6H5CH2OH. The benzyl group is often abbreviated "Bn" (not to be confused with "Bz" which is used for benzoyl), thus benzyl alcohol is denoted as BnOH. Benzyl alcohol is a colorless liquid with a mild pleasant aromatic odor. It is a useful solvent due to its polarity, low toxicity, and low vapor pressure. Benzyl alcohol has moderate solubility in water (4 g/100 mL) and is miscible in alcohols and diethyl ether. The anion produced by deprotonation of the alcohol group is known as benzylate or benzyloxide.

In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure C6H5CH2–. Benzyl features a benzene ring attached to a CH2 group.

Benzyl benzoate is an organic compound which is used as a medication and insect repellent. As a medication it is used to treat scabies and lice. For scabies either permethrin or malathion is typically preferred. It is applied to the skin as a lotion. Typically two to three applications are needed. It is also present in Balsam of Peru, Tolu balsam, and in a number of flowers.

Galeola is a genus of orchids belonging to the subfamily Vanilloideae.
Salicylic aldehyde (2-hydroxybenzaldehyde) is the organic compound with the formula C6H4CHO-2-OH. Along with 3-hydroxybenzaldehyde and 4-hydroxybenzaldehyde, it is one of the three isomers of hydroxybenzaldehyde. This colorless oily liquid has a bitter almond odor at higher concentration. Salicylaldehyde is a key precursor to a variety chelating agents, some of which are commercially important.
This is the list of extremely hazardous substances defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act. The list can be found as an appendix to 40 C.F.R. 355. Updates as of 2006 can be seen on the Federal Register, 71 FR 47121.

Gastrodia elata is a saprophytic perennial herb in the family Orchidaceae. It is found in Nepal, Bhutan, India, Japan, North Korea, Siberia, Taiwan, and China.
In enzymology, an aryl-alcohol dehydrogenase (EC 1.1.1.90) is an enzyme that catalyzes the chemical reaction
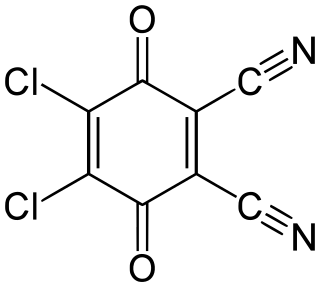
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (or DDQ) is the chemical reagent with formula C6Cl2(CN)2O2. This oxidant is useful for the dehydrogenation of alcohols, phenols, and steroid ketones in organic chemistry. DDQ decomposes in water, but is stable in aqueous mineral acid.
4-Hydroxybenzaldehyde is one of the three isomers of hydroxybenzaldehyde. It can be found in the orchids Gastrodia elata, Galeola faberi, and the Vanilla orchids.

In biochemistry, naturally occurring phenols refers to phenol functional group that is found in natural products. Phenolic compounds are produced by plants and microorganisms. Organisms sometimes synthesize phenolic compounds in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research. Some phenols are germicidal and are used in formulating disinfectants.

Gastrodin is a chemical compound which is the glucoside of gastrodigenin. It has been isolated from the orchid Gastrodia elata and from the rhizome of Galeola faberi. It can also be produced by biotransformation of 4-hydroxybenzaldehyde by Datura tatula cell cultures.
2,4-Bis(4-hydroxybenzyl)phenol is a phenolic compound produced by the saprophytic orchid Gastrodia elata and by the myco-heterotroph orchid Galeola faberi.

Dihydrostilbenoids (bibenzyls) are natural phenols formed from the dihydrostilbene (bibenzyl) backbone.

Trichloroacetonitrile is an organic compound with the formula CCl3CN. It is a colourless liquid, although commercial samples often are brownish. It is used commercially as a precursor to the fungicide etridiazole. It is prepared by dehydration of trichloroacetamide. As a bifunctional compound, trichloroacetonitrile can react at both the trichloromethyl and the nitrile group. The electron-withdrawing effect of the trichloromethyl group activates the nitrile group for nucleophilic additions. The high reactivity makes trichloroacetonitrile a versatile reagent, but also causes its susceptibility towards hydrolysis.

Homoisoflavonoids (3-benzylidenechroman-4-ones) are a type of phenolic compounds occurring naturally in plants.
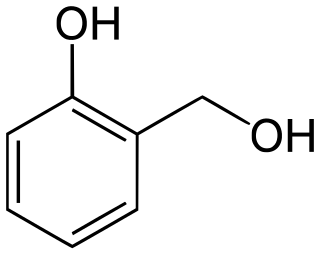
Salicyl alcohol (saligenin) is an organic compound with the formula C6H4(OH)(CH2OH). It is a white solid that is used as a precursor in organic synthesis.













